Abstract
Background: Approximately 20–40% of pts with NDMM present with RI, which is associated with a negative impact on survival (Rajkumar, 2005). In the pivotal phase 3 FIRST trial (median follow-up 37 months [mos]), continuous Rd improved progression-free survival (PFS) vs. melphalan-prednisone-thalidomide (MPT) in elderly NDMM pts by 28% (25.5 vs. 20.7 mos; HR = 0.72; P < 0.01) (Facon, Blood 2013). Although 121 pts receiving continuous Rd are still on Tx, the interim overall survival (OS) analysis showed a 22% reduction in the risk of death in favor of continuous Rd vs. MPT (HR = 0.78; P = 0.02). The present analysis was conducted to determine the impact of RI on PFS, OS, and time to 2nd antimyeloma Tx (AMT) as clinical study outcomes.
Methods: Pts were randomized to 3 Tx arms: continuous Rd until progression (n = 535); Rd for 18 cycles (72 weeks) (Rd18; n = 541); or MPT for 12 cycles (72 weeks) (n = 547). Enrolled NDMM pts were categorized according to their renal function: 24% had normal renal function (creatinine clearance [CrCl] ≥ 80 mL/min), 44% presented with mild RI (≥ 50 and < 80 mL/min), 23% had moderate RI (≥ 30 and < 50 mL/min), and 9% had severe RI (< 30 mL/min). Pts requiring dialysis were excluded. Lenalidomide starting dose was 25 mg QD for pts with normal renal function or mild RI, 10 mg QD for moderate RI, and 15 mg QOD for severe RI. Melphalan dose was reduced by 50% in pts with moderate or severe RI. The primary endpoint was PFS (continuous Rd vs. MPT); secondary endpoints were OS, overall response rate, time to response, duration of response, time to Tx failure, time to 2nd AMT, health-related quality of life, safety, and improvement in renal function from baseline. Improvement in RI was defined as shifts from baseline to most extreme post-baseline value of the calculated CrCl as a measure of renal function during the active Tx (N = 1484).
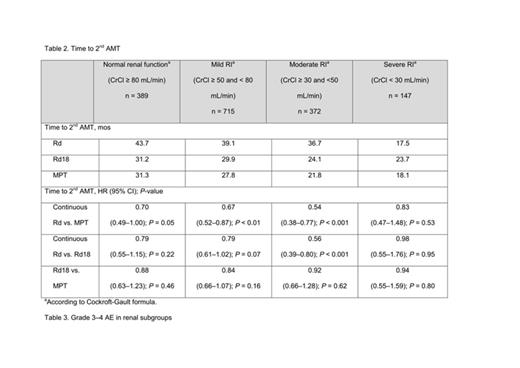
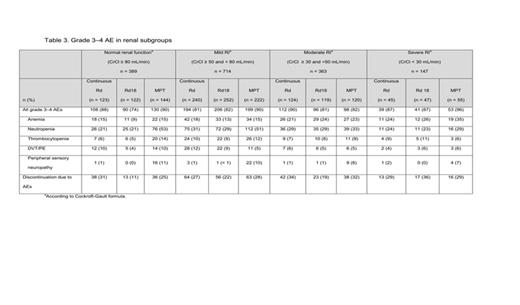
Results: A PFS benefit favored continuous Rd vs. MPT irrespective of the degree of renal function (Table 1): there was a benefit in pts with normal renal function (HR = 0.72 (0.51–1.02); P = 0.06), and better in pts with mild RI (HR = 0.79 (0.62–1.00); P = 0.05) and moderate RI (HR = 0.62 (0.45–0.85); P < 0.01). A PFS benefit was also seen with continuous Rd vs. Rd18 (a secondary comparison) in pts with mild RI and moderate RI (P < 0.01 for both). An interim OS benefit with continuous Rd vs. MPT was observed in most renal subgroups. Similar results were observed between Rd18 and MPT in terms of PFS or interim OS in any of the renal subgroups. Continuous Rd, compared with Rd18 or MPT, extended time to 2nd AMT in most renal groups except severe RI (CrCl < 30mL/min) (Table 2). Improvement in RI was observed more frequently in pts treated with continuous Rd than those with Rd18 or MPT: improvement of mild RI, 48%, 43%, and 48%, respectively; of moderate RI, 67% 61%, and 62%; and of severe RI, 64%, 59%, and 56%. Overall, < 5% of pts in any Tx group experienced a worsening in renal function status during Tx (continuous Rd 2.2%; Rd18 2.8%; MPT 2.7%). The most common grade 3–4 adverse events (AEs) for these Txs were anemia, neutropenia, thrombocytopenia, deep-vein thrombosis/pulmonary embolism (DVT/PE), and peripheral sensory neuropathy (Table 3). Tx discontinuation due to AEs increased in pts with moderate and severe RI, regardless of the type of Tx (Table 3).
Conclusions: PFS, OS (at interim analysis), and time to 2nd AMT outcomes generally improved continuous Rd vs. Rd18 or MPT in transplant-ineligible NDMM pts with normal renal function, and in those with mild or moderate RI. The small number of pts in the severe RI group precluded a meaningful conclusion. Continuous Rd was generally well tolerated and renal function improved in the majority of pts during Tx with continuous Rd vs. Rd18 or MPT.
Dimopoulos:Celgene Corporation: Consultancy, Honoraria. Off Label Use: Lenalidomide used in newly diagnosed multiple myeloma patients. Roussel:Celgene: Consultancy, Lecture fees Other, Research Funding. van der Jagt:Celgene Corporation: Research Funding. Jaccard:Celgene Corporation: Honoraria, Research Funding. Tosikyan:Celgene: Consultancy. Karlin:Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees; Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees; Sandoz: Honoraria, Membership on an entity's Board of Directors or advisory committees. Bensinger:Celgene Corporation: Consultancy, Research Funding. Schots:Celgene: Research Funding. Chen:Celgene Corporation: Employment. Marek:Celgene Corporation: Employment, Equity Ownership. Ervin-Haynes:Celgene Corporation: Employment. Facon:Celgene: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal