Abstract
Introduction: The prognosis of multiple myeloma (MM) depends on the underlying cytogenetic subtype. Recurrent cytogenetic changes including hyperdiploidy of odd-numbered chromosomes, -13/13q- and balanced translocations involving IGH have already been incorporated into risk stratification strategies and are used in standard clinical practice. Nevertheless, MM often exhibits a heterogenous and complex karyotype, and many other chromosomal abnormalities of uncertain clinical significance are observed. We performed a comprehensive analysis for the relationship between cytogenetic abnormalities of undetermined prognostic significance and known prognostic factors in MM.
Materials and Methods: Conventional G-banding was performed in 333 newly diagnosed MM. Karyotypes were described using the most recent version of the International System for Human Cytogenetic Nomenclature. Translocations with breakpoints at p10, p11, q10, or q11 were all regarded as whole arm (centromere to centromere) translocations. Poor prognostic indicators as follows: hemoglobin (Hb) level < 10 g/dL, calcium level > 12 mg/dL, IgG level > 7 g/dL or IgA level > 5 g/dL, b2-microglobulin level > 5.5 mg/L, and albumin level < 3.5 g/dL. Creatinine level > 2 mg/dL, IgA type, lambda type, elevated free light chain level > 100 mg/dL, abnormal kappa/lambda ratio of > 4.0 or < 0.5, and elevated LDH level were also included.
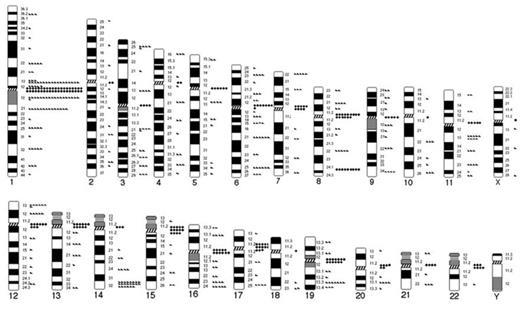
Results: Among the 333 MM patients, 212 showed a normal or good/intermediate karyotype (195 with normal karyotype; 17 with abnormal karyotype associated with good or intermediate prognosis); these served as the control group in this study. The remaining 121 had other cytogenetic abnormalities (with or without hyperdiploidy, or translocations associated with good or intermediate prognosis). Chromosomes 1p (12.0%), 1q (18.9%), 6q (7.2%), 8q (6.9%), 11q (5.4%), 12p (5.1%), 14q (9.3%) and 19q (5.1%).showed frequent abnormalities. Common breakpoints were p10–12, p13, p22 in 1p, q10–12, q21, q25, q32 in 1q; q21, q25 in 6q; q10, q22, q24 in 8q; q10, q13 in 11q; p10–12 in 12p; q24, q32in 14q and q10, q13.3 in 19q (Figure 1). Certain chromosomal abnormalities were associated with known prognostic factors (p-value < 0.05): 8q - IgA type; 1q - IgG level of > 5 g/dL or IgA level of> 3 g/dL; 14q - kappa or lambda type chains > 100 mg/dL; 1p, 1q, 6q, and 19q - Hb < 10.0 g/dL; 1p and 12q - albumin < 3.5 g/dL; 14q and 19q –creatinine > 2.0 mg/dL; 1p, 1q, 6q, or 19q - b2-microglobulin > 5.5 mg/L; 1p, 8q, 11q, and 14q - calcium > 12 mg/dL; 1p, 3p, 12p, or 14q – elevated LDH level.
Conclusion: We analyzed the distribution and clinical significance of the less commonly occurring cytogenetic abnormalities for the first time. Abnormalities in chromosomes 1p, 1q, 3p, 6q, 8q, 11q, 12q, 14q, and 19q were associated with clinical factors associated with a poor prognosis. Not all chromosomes showing frequent aberrations correlated with prognostic factors, indicating that it is not the presence of a cytogenetic abnormality in itself, but the particular chromosome(s) involved in the abnormality that is the key factor that determines the initial clinical characteristics of MM.
Diagrammatic representation of the chromosomal breakpoints involved in structural abnormalities in multiple myeloma. Triangles represent breakpoints in interstitial or telomeric regions and diamonds represent breakpoints in centromeric or pericentromeric (p10-p11 or q10-q11) regions.
Diagrammatic representation of the chromosomal breakpoints involved in structural abnormalities in multiple myeloma. Triangles represent breakpoints in interstitial or telomeric regions and diamonds represent breakpoints in centromeric or pericentromeric (p10-p11 or q10-q11) regions.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal