Abstract
Background: Assessment of malignant plasma cell cycling via plasma cell labeling index (PCLI) has been a validated prognostic tool in multiple myeloma (MM) but the test requires specialized technical expertise and is not widely available. Ki67 is a well-known protein marker of cellular proliferation on immunohistochemical (IHC) staining with prognostic utility in other malignancies. In an effort to develop a simpler system to provide analogous information to PCLI, we used a novel IHC co-staining technique for CD138 and Ki67 to quantify plasma cells in active cycling. We then performed a retrospective analysis of the ratio of Ki67/CD138 (Ki67%) in newly diagnosed patients with multiple myeloma receiving 1st-line therapy to correlate with clinical outcomes.
Methods: A retrospective cohort study of patients (pts) with treated symptomatic MM was performed by interrogation of the clinical database at the Weill Cornell Medical College / New York Presbyterian Hospital. For inclusion in the analysis, subjects must have started first-line treatment in the period of 2005-2010, and had available bone marrow biopsies. Double-staining with Ki67 and CD138 was performed by IHC. The Ki67% was calculated as the percent of plasma cells expressing CD138 that were also found to express Ki67. Treatment outcomes were stratified and compared based on %Ki67. Response was determined by monthly serum protein electrophoresis / immunofixation (IFX) with free light chain analysis according to International Multiple Myeloma Working Group (IMWG) guidelines. Pts who were IFX negative but had no subsequent bone marrow biopsy were classified as being in unconfirmed complete remission.
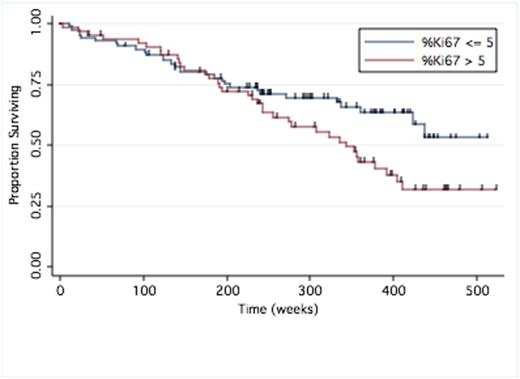
Results: We identified 151 patients with newly diagnosed MM and available %Ki67 expression who received first-line therapy over the period of 2005-2010. Patient were subdivided into two groups based on %Ki67: Low: %ki67 <= 5%, n = 87; and High: %Ki67 >5, n=64, to allow for comparison of treatment response and survival analysis. Specific therapeutic agent exposure history did not differ significantly between patients. Both groups had similar depth of response rates (ORR) to front-line therapy, Table 1. Median progression-free survival for the high versus low %Ki67 groups approached statistical significance at 54 months (95% CI 30.8,67.4) versus 26.9 months (95% CI 21.6,40.2), respectively (P = 0.083). At data cut-off, there were 30 deaths in the low %Ki67 group (1-yr OS 93%, 5-yr OS 71%) and 36 deaths in the high %Ki67 group (1-yr OS 94%, 5-yr OS 62%). Median overall survival (OS) was not reached for Ki67% <= 5% (95% CI 97.3,NR) vs. 78.9 months (95% CI 55.9,93.1) for Ki67% > 5%, (P = 0.0434), Figure 1. Multivariate cox regression for factors with influence on OS showed that only high-risk cytogenetics (HR 2.05, 95% CI 1.17, 2.92, P = 0.027), ISS (HR 1.835, 95% CI 1.33, 3.60, P = 0.000), and %Ki67 group status had an independent effect on survival outcome. Low (<=5%) versus high (>5%) %Ki67 influenced overall survival with a hazard ratio of 1.76 (CI 1.07,2.92, P = 0.027). Survival after ASCT was significantly longer in the low %Ki67 group with median OS not reached (95%CI, 97.3, NR) versus 86.9 months (95% CI 43.9, NR) for high %Ki67 group (P = 0.04).
Discussion: The ratio of IHC double positive Ki67 and CD138 of > 5% is an independent prognostic marker for overall survival in newly diagnosed MM undergoing 1st line therapy. The %Ki67 serves as a simpler and widely available analog to PCLI that can be presently performed in most hematopathology laboratories.
First Line Treatment and Best Response (modified IMWG Criteria)
| . | Ki67% <= 5 (N = 87) n (%) . | Ki67% > 5 (N = 64) n (%) . | P . |
|---|---|---|---|
| Treatment Exposure* | |||
| Lenalidomide | 59 (67.8) | 48 (75) | 0.34 |
| Thalidomide | 30 (34.5) | 14 (21.9) | 0.09 |
| Bortezomib | 25 (28.7) | 14 (21.9) | 0.34 |
| Alkylating agent | 11 (12.6) | 4 (6.3) | 0.19 |
| ASCT | 27 (31) | 22 (34.4) | 0.66 |
| Best Response | |||
| Overall Response (>= Partial response) | 77 (88.4) | 57 (89.1) | 0.41 |
| Complete response | 15 (17.2) | 22 (34.4) | |
| Unconfirmed complete response** | 14 (16.1) | 8 (12.5) | |
| Very good partial response | 23 (26.4) | 15 (23.4) | |
| Partial response | 25 (28.7) | 12 (18.8) | |
| Stable disease | 9 (10.3) | 5 (7.8) | |
| Progressive disease | 1 (1.2) | 2 (3.1) |
| . | Ki67% <= 5 (N = 87) n (%) . | Ki67% > 5 (N = 64) n (%) . | P . |
|---|---|---|---|
| Treatment Exposure* | |||
| Lenalidomide | 59 (67.8) | 48 (75) | 0.34 |
| Thalidomide | 30 (34.5) | 14 (21.9) | 0.09 |
| Bortezomib | 25 (28.7) | 14 (21.9) | 0.34 |
| Alkylating agent | 11 (12.6) | 4 (6.3) | 0.19 |
| ASCT | 27 (31) | 22 (34.4) | 0.66 |
| Best Response | |||
| Overall Response (>= Partial response) | 77 (88.4) | 57 (89.1) | 0.41 |
| Complete response | 15 (17.2) | 22 (34.4) | |
| Unconfirmed complete response** | 14 (16.1) | 8 (12.5) | |
| Very good partial response | 23 (26.4) | 15 (23.4) | |
| Partial response | 25 (28.7) | 12 (18.8) | |
| Stable disease | 9 (10.3) | 5 (7.8) | |
| Progressive disease | 1 (1.2) | 2 (3.1) |
* Percentages do not add to 100% due to instances of concurrent therapy use
** Unconfirmed complete response: immunofixation negative, but no confirmatory bone marrow biopsy available
Overall Survival by %Ki67
Mark:Celgene: Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Millennium: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Onyx: Research Funding, Speakers Bureau. Rossi:Celgene: Speakers Bureau. Pekle:Celgene: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Millennium: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau. Perry:Celgene: Speakers Bureau. Coleman:Celgene: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Millennium: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Onyx: Honoraria. Niesvizky:Celgene: Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Millennium: Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Onyx: Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal