Abstract
Background: Hemophilia is an inherited condition, requiring lifelong, expensive treatment driven primarily by the cost of medications. Initiating prophylaxis treatment with factors VIII (hemophilia A) or IX (hemophilia B) at an early age has been shown to be effective in reducing the frequency of bleeding episodes and preventing hemophilic arthropathy. Consequently, in 2007 the Medical and Scientific Advisory Council MASAC) of the National Hemophilia Foundation (NHF) recommended prophylaxis treatment as the optimal therapy for these patients. Although several studies have calculated the cost of caring for a hemophilia patient based on the population as a whole, no studies to date have explored changes in costs over a patient's lifespan or quantified how the evolution of clinical guidelines have affected these costs.
Aims: (1) To explore differences in the economic burden of treating hemophilia A/B over the patient's lifespan; (2) To quantify changes in factor utilization and related costs over the past decade.
Methods: A retrospective analysis of US health insurance claim database (Truven Health Analytics) collected between January 2004 and December 2012 was conducted. Males with ≥2 pharmacy claims for a hemophilia drug (identified by National Drug Codes) within 3 months, and continuous enrollment for ≥180 days were included. Patients utilizing inhibitor treatments were excluded. Annual payer cost and patient out-of-pocket (OOP) expenses were calculated by service category (inpatient, outpatient, medications), and were further stratified by patient's age and calendar year of service. All costs were adjusted to 2012 USD$ values. To understand trends in factor utilization, pharmacy claims data were used to calculate annual supply days per patient (prescribed total day's supply per patient divided by the patient's total enrollment days multiplied by 365). First vs. last year supply days were compared using a t-test.
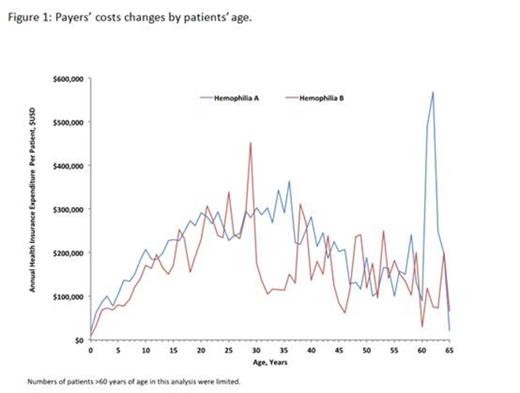
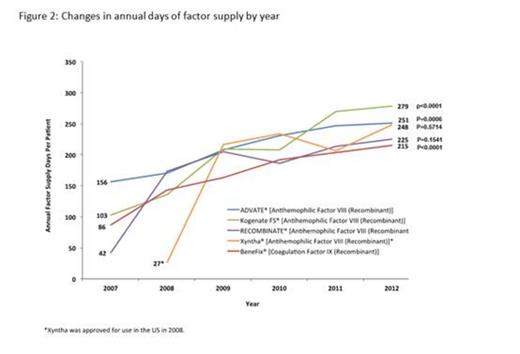
Results: A total of 626 hemophilia A patients and 136 hemophilia B patients met the inclusion criteria. A steady increase in payers' costs was observed during the first 3 decades of life, with peak annual cost at age 36 for hemophilia A patients ($363,948) and at age 29 for hemophilia B patients ($453,179), followed by a decrease in costs during the 4th and 5th decade of life (Figure 1). Annual per patient OOP expenses showed little variation by age, with the mean per patient OOP cost averaging $2,672/year for hemophilia A, and $1,838/year for hemophilia B. As shown in Figure 2, between 2007 and 2012, annual days supply of factor replacement per patient increased significantly (p<0.05) for both factor VIII (e.g., Advate®: from 156.5 days in 2007 to 251 days in 2012, p=0.0006) and factor IX products (e.g., BeneFIX®: from 86 days in 2007 to 215 days in 2012, p<0.0001), suggesting increased annual factor utilization over the analysis period. A trend towards increasing payer cost for drugs dispensed to both hemophilia A patients (2007: $155,239 to 2012:$206,027) and hemophilia B patients (2007: $129,002 to 2012: $179,747) was observed. Additionally, a trend towards decreased outpatient cost over time for hemophilia A patients (2007: $66,710 to 2012: $34,571) and increased inpatient cost (2007: $41,981 to 2012: $57,028) was observed. No specific trends were observed for hemophilia B patients across outpatient (2007:$32,762 to 2012: $34,702) or inpatient costs (2007:$82,072 to $109,401).
Summary/Conclusion: Over the past decade, factor utilization has increased substantially among hemophilia A and B patients, with payers assuming most of the additional costs. These changes in utilization may indicate that the hemophilia population is increasingly receiving prophylactic therapy in line with the 2007 NHF MASAC recommendations. Surprisingly, costs associated with increased drug consumption were not consistently offset by decreases in inpatient or outpatient services. The overall per patient OOP expenses per year have remained steady and predictable over time and over the patient's lifespan possibly due to imposed annual limits on OOP by insurance policies. As newer treatments for Hemophilia A and B become available in the market, additional evaluation will be necessary to understand any implications of these agents on the downstream medical utilization and costs.
Eldar-Lissai:Biogen Idec: Employment, Equity Ownership. Hou:Biogen Idec: Employment, Equity Ownership. Krishnan:Biogen Idec: Employment, Equity Ownership.
Author notes
Asterisk with author names denotes non-ASH members.
This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal