Abstract
Introduction: Ibrutinib is an irreversible first-in-class inhibitor of BTK (Bruton tyrosine kinase) approved for the therapy of mantle cell lymphoma (MCL) and chronic lymphocytic leukemia (CLL). Ongoing or planned clinical studies evaluate the combination of ibrutinib the CD20 antibodies rituximab or obinutuzumab (GA101) in CLL (first-line and relapsed). However, some concerns have emerged due to published preclinical data showing that ibrutinib can interfere with efficacy of therapeutic antibodies in vitro and in vivo through two main mechanisms, (i) inhibition of NK-dependent ADCC through specific targeting of BTK/ITK signaling in effector cells (Kohrt et al., Blood, 2014 Mar 20;123(12):1957-60), and (ii) through modulation of CD20 expression at the surface of tumor target cells (Bojarczuk B et al. Leukemia 2014;28:1163-67). Nevertheless, clinical trials show an increased overall response rate comparing rituximab+ibrutinib and ibrutinib alone (Burger J., ASH 2013, Abstract 675).
Two intriguing points prompted us to conduct our current study: (i) 0.1-1microM ibrutinib doses were used in these published data, and in phase I studies, mean peak concentration of ibrutinib 420mg/d in CLL patients were 72ng/ml (0.16microM), and (ii) loss of CD20 expression recovered rapidly after wash-out periods. We therefore evaluated B-cell depletion induced by rituximab (RTX) and obinutuzumab (GA101) in vitro, using peripheral blood mononuclear cell (PBMC) samples taken from relapsed CLL patients (pts) under ibrutinib monotherapy (420mg/d).
Methods: In 8 pts (median prior lines=4, range=2-8), PBMCs were collected before ibrutinib initiation and after 1 and 2 months of therapy. PBMC were seeded at 10 x 106 cells/mL in culture medium and treated for 7 days with 10µg/mL control IgG1 (trastuzumab), rituximab or GA101. The specific percentage of remaining B cells in monoclonal antibodies–treated samples was calculated as (absolute number in treated samples/absolute number in control samples) x 100. For each condition, the absolute number of remaining B cells was calculated as total viable cell number (trypan blue exclusion determination) x % of viable CD19+/CD5+ lymphocytes (flow cytometry determination). For statistical analyses, Student's test (paired, two-sided) was used.
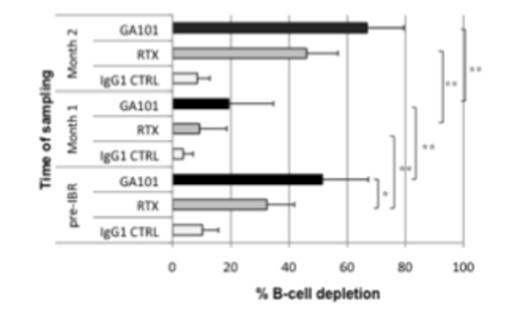
Results:In patients prior to start of ibrutinib treatment, GA101-induced B cell depletion was significantly higher than that observed with rituximab (mean 51.4% vs 32.4%) as shown in Figure 1 (**p<0.01; *p<0.05). After 1 month of ibrutinib exposure, hyperlymphocytosis and spleen/lymph node shrinkage were noticed in 8/8 pts. Samples were collected to re-assess ADCC using the same treatment conditions. Both, GA101- and rituximab-mediated B-cell depletion were significantly reduced (RTX: mean 32.4% vs 9.3%, GA101: 51.4% vs 19.3%), thus confirming previously published in vitro data suggesting ibrutinib may indeed have a detrimental effect on rituximab, but may also affect B cell depletion mediated by GA101. Importantly, for the first time, these effects were evaluated with physiological doses of ibrutinib in each sample (as derived from patients under treatment), in a 100% autologous system, with relevant effector:target ratio. Yet, this decrease of ADCC efficacy was unlikely explained by the nature of target cells (newly mobilized CLL cells that may be more refractory to therapy-refractory), the decreased E:T ratio (induced by mobilizing CLL cells, whereas there was no significant expansion of NK cells under ibrutinib therapy, data not shown), or ibrutinib dose. Indeed, when samples were collected at month 2 when pts still had high lymphocyte counts to monitor ADCC efficacy, we observed a strong restoration of both rituximab- and GA101-mediated B-cell depletion (RTX: mean 46% vs 9.3%, GA101: 66.8% vs 19.3%). One hypothesis, currently addressed in our lab, is that NK cells may become independent from ITK signaling and thus recover full efficacy after 2 months of ibrutinib therapy.
Conclusions: Previous preclinical investigations suggesting antagonistic effects between rituximab and ibrutinib were confirmed by our tests using ibrutinib-exposed PBMCs from CLL patients under ibrutinib treatment. Yet, over time a significant recovery of ADCC over time was observed, and since complement components are left unaffected by ibrutinib, the combination of ibrutinib and rituximab or GA101 are supported.
Klein:Roche: Employment, Equity Ownership, Patents & Royalties.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal