Abstract
Introduction: CD23 is a 45-kDa transmembrane glycoprotein corresponding to the low-affinity receptor for the immunoglobulin E (IgE). There are two isoforms - CD23a expressed on B-cells, and CD23b primarily expressed on monocytes, T-cells and platelets. The CD23 protein can be cleaved and released into the serum as a soluble form of CD23 (sCD23). The sCD23 is a 25kDa fragment that can be found in serum, plasma and urine in patients with chronic lymphocytic leukemia.
Objective: To evaluate a novel and robust method of sCD23 detection using CBA detection, and correlate with known markers of prognostication and clinical outcome.
Methods: At Barts & The London School of Medicine, historical serum samples have been taken at diagnosis from patients with CLL between 1984 to 2012 with corresponding clinical data. Serum analysis was obtained from 60 healthy controls and assessed for sCD23 in ng/ml (France and Canada).
sCD23 levels (ng/ml) were evaluated against known prognostic markers and clinical features, including: ZAP-70 (>20%), CD38 (>20%), B2M, cytogenetics (17p-/11q- vs 12+/13q-/normal), LDH, splenomegaly, hepatomegaly, and lymphadenopathy.
107 samples from 86 patients underwent retrospective assessment of sCD23 from stored serum. The sCD23 cytometric bead array (CBA) flow cytometric assay (BD Biosciences) captures the soluble analyte with beads of known size and fluorescence.1 Capture beads are identified and sCD23 quantification (in ng/ml) is performed based on PE fluorescence excitation. Results were acquired using FACSDiva (BD Biosciences) and analysed using FCAParray. Statistical analysis was performed and significance was set at <0.05%.
Results: sCD23 levels from the 86 patients ranged from 1.92 to 2441 ng/ml with a median value of 234.5 ng/ml. The sCD23 levels from the healthy control group ranged from 0.43 to 5.16 ng/ml with a median value of 1.56 ng/ml (figure 1).
sCD23 levels between healthy controls and patients
Within the CLL patient cohort, elevated sCD23 significantly correlated with 6 out of the 8 prognostic & clinical indicators (Table 1).
Median sCD23 (ng/ml) for CLL patients by prognostic risk stratification.
| Indicator . | Median sCD23 (ng/ml) . | Level of significance (MWU-test) . |
|---|---|---|
| ZAP-70 positive (n=19) | 1,096.0 | P<0.01 |
| ZAP-70 negative (n=38) | 116.3 | |
| CD38 positive (n=22) | 389.5 | P=0.06 |
| CD38 negative (n=50) | 114.1 | |
| Poor risk karyotype (n=44) | 580.7 | P<0.01 |
| Good risk karyotype (n=38) | 135.8 | |
| B2M high (n=25) | 116.9 | P=0.95 |
| B2M normal (n=16) | 200.7 | |
| LDH high (n=31) | 561.7 | P=0.03 |
| LDH normal (n=34) | 140.6 | |
| Lymphadenopathy (n=50) | 390.2 | P<0.01 |
| No lymphadenopathy (n=25) | 40.80 | |
| Splenomegaly (n=30) | 875.2 | P<0.01 |
| No splenomegaly (n=41) | 73.60 | |
| Hepatomegaly (n=11) | 425.8 | P=0.04 |
| Hepatomegaly (n=44) | 110.2 |
| Indicator . | Median sCD23 (ng/ml) . | Level of significance (MWU-test) . |
|---|---|---|
| ZAP-70 positive (n=19) | 1,096.0 | P<0.01 |
| ZAP-70 negative (n=38) | 116.3 | |
| CD38 positive (n=22) | 389.5 | P=0.06 |
| CD38 negative (n=50) | 114.1 | |
| Poor risk karyotype (n=44) | 580.7 | P<0.01 |
| Good risk karyotype (n=38) | 135.8 | |
| B2M high (n=25) | 116.9 | P=0.95 |
| B2M normal (n=16) | 200.7 | |
| LDH high (n=31) | 561.7 | P=0.03 |
| LDH normal (n=34) | 140.6 | |
| Lymphadenopathy (n=50) | 390.2 | P<0.01 |
| No lymphadenopathy (n=25) | 40.80 | |
| Splenomegaly (n=30) | 875.2 | P<0.01 |
| No splenomegaly (n=41) | 73.60 | |
| Hepatomegaly (n=11) | 425.8 | P=0.04 |
| Hepatomegaly (n=44) | 110.2 |
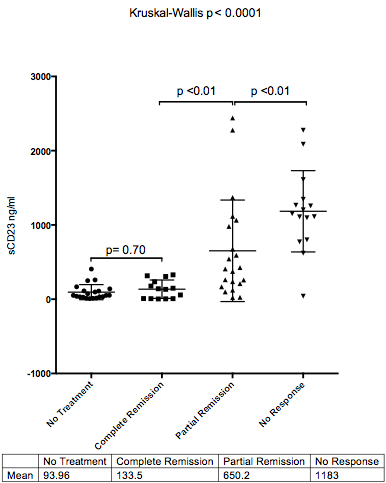
In addition, there was a strong correlation with response to therapy. Those patients who never required therapy (n=21) had significantly lower levels of sCD23 (mean 93.36ng/ml) over those who achieved a partial remission (n=21, mean 650.2 ng/ml p<0.01), or those with no response to therapy (n=15, mean 1183.0 ng/ml, p<0.01). There was no difference between those with never required therapy and those who achieved a complete remission (n=14, mean 133.5ng/ml, p=0.70) (Figure 2).
Mean sCD23 level (ng/ml) at diagnosis against response to therapy when required.
Mean sCD23 level (ng/ml) at diagnosis against response to therapy when required.
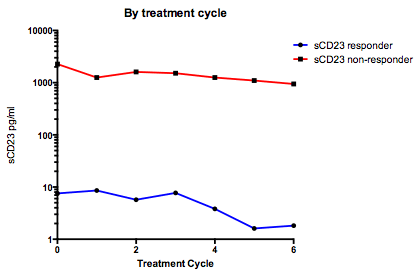
Two patients had sequential serum samples taken throughout therapy. The patient with elevated sCD23 at diagnosis remained elevated through each treatment cycle to only achieve partial response, whilst the patient with low sCD23 at diagnosis responded well to therapy and achieved a complete remission (p=0.01, Figure 3).
sCD23 following therapy. sCD23 non-responder did not respond to FC or FCR therapy . The responder received Chlorambucil+R then FCR.
sCD23 following therapy. sCD23 non-responder did not respond to FC or FCR therapy . The responder received Chlorambucil+R then FCR.
Conclusion: We have evaluated in a pilot study the sCD23 CBA assay, which is an easy and reproducible assay. Elevated levels of sCD23 at diagnosis correlated with “physical” prognosis, as well as known biological markers of prognostication. Those patients with elevated levels of sCD23 at diagnosis correlated with response to therapy regardless of therapy given, and there is evidence that patients who do not respond to therapy retain their elevated levels of sCD23 during therapy. This simple assay could provide the basis for individualised risk stratification and prediction of response to therapy based on an individual analyte.
Ref:
1 Grelier A et al. Cytometry B. 2014 Mar;86(2):91-7.
Farren:BD Biosciences: Research Funding. Nauwelaers:BD Biosciences: Employment. Keenan:BD Bioscience: Employment. Warner:BD Biosciences: Employment. Agrawal:BD Biosciences: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal