Abstract
Background: The choice of optimal salvage therapy in patients with MDS is challenging due to a current lack of therapeutic options and a lack of data in regard to the risks and benefits of existing disease management. We undertook this study with the aim of evaluating the clinical outcomes, economic impact, and cost effectiveness of currently available treatment options for MDS patients who failed first-line hypomethylating agent (HMA) therapy.
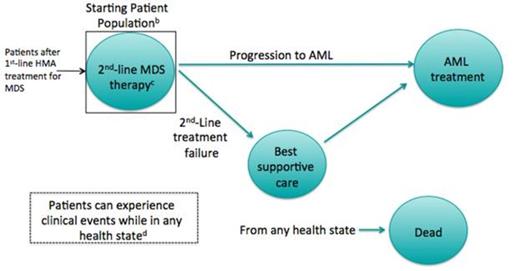
Methods: We developed a Markov model to compare five second-line MDS treatment options: low- and high-intensity chemotherapy; switching HMA treatment; hematopoietic stem cell transplant (HSCT); and best supportive care (BSC). Hypothetical cohorts of patients who had failed a first-line HMA were simulated, and initiated each of these strategies. During each four-week cycle, patients could progress to acute myeloid leukemia (AML), experience a treatment- or disease-related adverse event (thrombocytopenia, anemia and neutropenia), discontinue treatment, or die (Figure 1). Costs were considered from the payer perspective and included those related to drug acquisition and administration, adverse event treatment, hospitalization, and supportive care. Inputs were based on published literature and expert opinion. Results were reported as lifetime costs (2013 USD), life expectancy in life years (LY), and incremental cost-effectiveness ratios (ICERs).
AML, acute myeloid leukemia; HMA, hypomethylating agent; MDS, myelodysplastic syndrome.
a Green circles represent model health states.
b MDS patients who have failed initial HMA therapy.
c Second-line treatments considered for model strategies/comparators are: best supportive care (BSC), HMA, low or high intensity chemotherapy, or hematopoietic stem cell transplant.
d Adverse events: thrombocytopenia, anemia, and neutropenia.
Results: The model predicted that treating patients who had failed a first-line HMA with BSC was the least expensive option ($55,343 per person) but provided the shortest survival: 0.48 years. Switching patients to a second HMA increased costs to $84,625 and could extend survival modestly. Patients treated with low- and high-intensity chemotherapy had lifetime costs of $89,877 and $146,519 and life expectancy of 0.88 and 1.08 years, respectively. HSCT patients had the highest lifetime costs ($492,359) and survival (2.26 years). Compared with BSC, the ICER for low intensity chemotherapy was $87,343/LY gained, while high intensity chemo and transplant had ICERs of $284,303 and $291,375/LY, respectively. The strategy of switching patients to a second HMA was removed during the calculation of ICERs due to extended dominance, i.e., there was another strategy, namely low intensity chemotherapy, that provided greater clinical benefit and had a more attractive ICER.
Conclusions: For MDS patients who had relapsed after, failed to respond, or progressed during administration of a first-line HMA, subsequent alternative active treatments provide some clinical benefit. However, such therapies substantially increase costs and a relatively small population of patients may be eligible due to functional status or matching donors. Although our results suggest that high intensity chemotherapy and transplant provide the greatest clinical benefits, they may cause substantial treatment-related morbidity. The increases in cost of care could thus be interpreted as an inefficient use of resources according to current societal standards, especially as the availability of these therapies is restricted to a limited number of patients. These findings suggest that there is an unmet need among MDS patients failing first-line HMA therapy; more research and treatment options would benefit clinical decision-making, patient outcomes, and healthcare resource allocation.
Cogle:Partnership for Health Analytic Research (PHAR): Consultancy, I was paid as a consultant for my expert opinion while developing the analyses Other. Mukherjee:Partnership for Health Analytic Research: Consultancy, I was paid as a consultant for my expert opinion while developing the analyses Other. Lawrence:Onconova Therapeutics, Inc. : Employment. McKearn:Onconova Therapeutics, Inc. : Employment. Percy:Onconova Therapeutics, Inc. : Employment. Petrone:Onconova Therapeutics, Inc. : Employment, Stock Options Other. Megaffin:Onconova Therapeutics, Inc. : Employment. Anene:Partnership for Health Analytic Research (PHAR): Employment, I am an employee of PHAR, LLC, which was paid by Onconova Therapeutics to conduct the analyses described in this abstract. Other. Ortendahl:Partnership for Health Analytic Research (PHAR): Employment, I am an employee of PHAR, LLC, which was paid by Onconova Therapeutics to conduct the analyses described in this abstract. Other. Romanus:Partnership for Health Analytic Research (PHAR): Employment, I am an employee of PHAR, LLC, which was paid by Onconova Therapeutics to conduct the analyses described in this abstract. Other. Bentley:Partnership for Health Analytic Research (PHAR): Employment, I am an employee of PHAR, LLC, which was paid by Onconova Therapeutics to conduct the analyses described in this abstract. Other.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal