Abstract
Introduction:
The chronic Philadelphia chromosome negative myeloproliferative neoplasms (MPNs), including polycythemia vera (PV), essential thrombocythemia (ET) and primary myelofibrosis (PMF), are clonal stem cell disorders characterized by unregulated stem cell expansion and overproduction of blood cells. The acquired mutation JAK2V617F plays a central role in the pathogenesis of MPN. Ruxolitinib was the first FDA-approved JAK2 inhibitor for the treatment of MPNs; however, its use is limited by various toxicities due to its suppression of normal marrow function. Because its toxicity is related to dosage, combinations of drugs may enhance the therapeutic effectiveness of Ruxolitinib and permit the administration of lower doses of Ruxolitinib.
Cancer cells depend on a continued supply of glucose and glutamine for cell survival and proliferation. Glutaminase (GLS) is the key enzyme in glutamine metabolism. We report here that JAK2V617F mutation is associated with increased cellular metabolism and the GLS inhibitor BPTES (Bis-2-(5-phenylacetamido-1,3,4-thiadiazol-2-yl)ethyl sulfide) improves the therapeutic efficacy of Ruxolitinib in both JAK2V617F-mutant cell lines and MPN patient samples.
Methods:
The JAK2V617F-mutant human erythroleukemia (HEL) cell line and the murine prolymphoid cell line Ba/F3 expressing wild-type or mutant JAK2 (BaF3-hEPOR-JAK2wt cell and BaF3-hEPOR-JAK2V617F cell) were maintained as previously described. Cells were treated with DMSO, BPTES (Sigma¨), and/or Ruxolitinib (Selleckchem¨) and cell proliferation was assessed by counting bright live cells in a hemocytometer using the trypan blue exclusion method. Mitochondrial respiration (oxygen consumption rate, OCR) and glycolysis (extracelluar acidification rate, ECAR) were measured using a Seahorse¨ XFe96 Bioanalyzer. We used 13C stable isotope analysis to determine differences in glucose and glutamine utilization. BaF3-hEPOR-JAK2wt cell and BaF3-hEPOR-JAK2V617F cell were grown in [U6]-13C glucose or [U5]-13C glutamine for 24 hours and metabolites extracted and prepared for GC-MS analysis. Data were analyzed using MassHunter software (Agilent Technologies¨).
MPN patient specimens were obtained with informed consent and approval by the IRB of Northport VA Medical Center. Peripheral blood CD34+ cells from MPN patients were isolated using immunomagnetic beads (Miltenyi¨) as we previously did. MPN CD34+ cells were treated with DMSO, BPTES, and/or Ruxolitinib and assayed in methylcellulose semisolid medium (Stem Cell Technologies¨) with growth factors. Colonies were counted after 14 days of incubation.
Results:
OCR and ECAR were increased by 55% (p < 0.0001) and 2-fold (p < 0.0001), respectively, in BaF3-hEPOR-JAK2V617F cells compared to BaF3-hEPOR-JAK2wt cells. This indicated that the JAK2V617F-mutant cells have higher metabolic activity than JAK2WT cells. Stable isotope analysis using GC/MS showed that JAK2V617F-mutant cells had increased utilization of glucose and glutamine into the TCA cycle as well as increased lactate production from glucose. There was a higher glutaminase activity in the JAK2V617F-mutant cells than the JAK2WT cells.
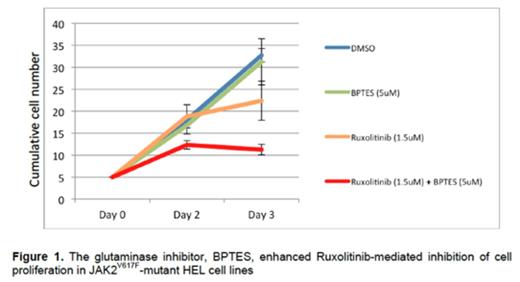
Next we determined the effect of the glutaminase inhibitor, BPTES, on Ruxolitinib-mediated inhibition of cell proliferation in JAK2V617F-mutant cell lines. While BPTES (5µM) had little effect on HEL cell growth, the combination of BPTES (5µM) and Ruxolitinib (1.5µM) significantly inhibited HEL cell growth by 66% while Ruxolitinib alone inhibited cell growth by only 35% (p = 0.04). Similar results were obtained with the BaF3-hEPOR-JAK2V617F cells. (Figure 1)
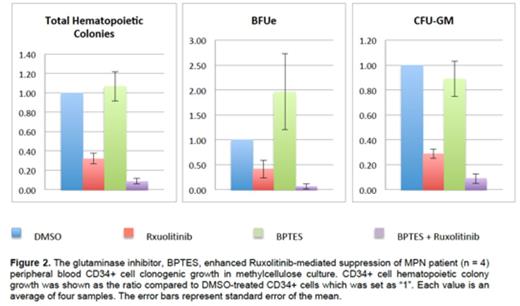
Finally, we tested the combination therapy of BPTES and Ruxolitinib in peripheral blood CD34+ cells from 4 MPN patients (2 PMF and 2 ET). The combination of BPTES (2uM) and Ruxolitinib (125nM) suppressed total hematopoietic colony growth of MPN CD34+ cells by 91% in comparison to 68% inhibition by Ruxolitinib alone (p = 0.007). Both CFU-GM colony and BFUe colony were suppressed by the combination therapy more than by Ruxolitinib alone although it was only significant for CFU-GM colony formation. (Figure 2)
Conclusion:
Our study showed that JAK2V617F mutation was associated with increased cellular metabolism. Importantly, a glutaminase inhibitor enhanced the therapeutic efficacy of Ruxolitinib in both JAK2V617F-mutant cell lines and MPN patient peripheral blood CD34+ cells.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal