Abstract
The JAK1/2 inhibitor ruxolitinib (RUX) is effective in decreasing symptomatic splenomegaly, and constitutional symptoms in patients with myelofibrosis (MF). Long-term data suggest that treatment with RUX is associated with a survival benefit, and may delay/reverse bone marrow fibrosis. Further, a ≥ 20% reduction in JAK2 V617F allele burden with RUX has been described (Vannucchi et al. Blood. 2012). Yet, many uncertainties surrounding the full clinical potential of RUX persist. The conceivable disease-modifying impact including the achievement of a JAK2 V617F molecular remission under sustained JAK1/JAK2 inhibition in a patient with primary myelofibrosis (PMF) is presented.
Patient and Methods: the diagnosis of JAK2 V617F-positive PMF according to the 2008 WHO criteria was made in a 50-years old caucasian male in February 2009. On the first consultation in November, 2009, he complained of fatigue and night sweats. Spleen was 12 cm below costal margin by palpation. Laboratory results were: Hb 153 g/L, WBC 18.9 x109/L, platelets 268 x109/L, peripheral blasts 2%, a leucoerythroblastic blood picture, LDH 10.6 µkat/L [2.8x upper limit of normal]. With an IPSS of 2 points (night sweats and peripheral blasts > 1%) the IPSS risk category for survival was intermediate-II. After signing informed consent, the patient was included in the phase 3, multicenter COMFORT-II trial and randomized to treatment with RUX at a dose of 20mg bid based on platelets count. By week (w) 4, the dose was increased to 25mg bid per protocol (< 40% reduction is palpable spleen length). For efficacy and safety evaluations, serial clinical, spleen volume [by magnetic resonance imaging (MRI)], blood picture, blood chemistry, bone marrow trephine biopsy, and JAK2 V617F allele burden from both peripheral blood samples as well as bone marrow assessments were conducted. The histology of the biopsies was evaluated according to the WHO criteria by experienced pathologists (Table). The JAK2 V617F allele burden was measured from blood samples using allele-specific oligonucleotide quantitative real-time polymerase chain reaction (qPCR) with a dynamic detection range from 0.1 to100% mutated allele (Lange et al. Haematologica 2013) and from bone marrow samples via qPCR followed by pyrosequencing for determining the allelic burden with a sensitivity of 5% of mutated DNA. If necessary, blocking of the non-mutated allele for sensitivity enhancement from 5% up to 0,001% of mutated DNA was done (Siebolts et al. J Clin Pathol 2010).
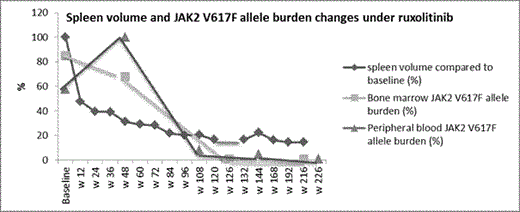
Results: After a follow-up of 240 weeks, treatment with 25mg bid is ongoing. No dose modification/interruption because of adverse events was required. Clinical response was rapid with a 52% reduction in baseline spleen volume from 3.77 L to 1.8 L and improvement in constitutional symptoms at w 12. This was followed by a further decline in spleen volume. By w 216, spleen volume was 0.5 L which corresponded to 85.5% reduction from baseline (Figure). WBC and LDH normalized by w 24 and w 84 respectively. Histologic improvement in marrow cellularity, megakaryocytic, and granulocytic lineages was first evident at w 126 with further reversal of MF-related abnormalities including marrow fibrosis by w 216 (Table). JAK2 V617F allele burden of < 10% in a peripheral blood sample and < 1% in the bone marrow were first documented at w 108 and w 132 respectively. As of w 216, an allele burden <1% was sustained in both blood and marrow samples (Figure). Conclusions: The rapid symptomatic improvement with ruxolitinib could be followed by a considerable MF-modifying response with the potential to alter the course of disease and prognosis. Unlike targeted therapy for chronic myeloid leukemia, ruxolitinib-delivered molecular and histologic remissions could be gradual, and progressive. Whether such deep long-term responses are dose-dependent is not yet known. Thus, the full potential of a sustained JAK1/JAK2 inhibition needs to be elucidated by carefully analyzing the possible correlation between dose, short-term spleen responses and long-term molecular and histologic responses; both in retro- and prospective studies.
Changes in marrow histology and spleen volume under ruxolitinib
| . | Marrow Cellularity % . | Megakaryocyte . | Granulocytic Proliferation . | G:E . | Marrow Fibrosis (EUMNET) . | Spleen volume (L) . | |
|---|---|---|---|---|---|---|---|
| Proliferation . | Atypia . | ||||||
| Baseline | 100% | +++ | +++ | +++ | 8:1 | PMF1 | 3.8 |
| Week 48 | 80% | ++ | +++ | ++ | 5:1 | PMF2 | 1.2 |
| Week 126 | 30% | + | ++ | - | 1:3 | PMF1 | 0.6 |
| Week 216 | 40% | - | + | - | 3:1 | PMF0 | 0.5 |
| . | Marrow Cellularity % . | Megakaryocyte . | Granulocytic Proliferation . | G:E . | Marrow Fibrosis (EUMNET) . | Spleen volume (L) . | |
|---|---|---|---|---|---|---|---|
| Proliferation . | Atypia . | ||||||
| Baseline | 100% | +++ | +++ | +++ | 8:1 | PMF1 | 3.8 |
| Week 48 | 80% | ++ | +++ | ++ | 5:1 | PMF2 | 1.2 |
| Week 126 | 30% | + | ++ | - | 1:3 | PMF1 | 0.6 |
| Week 216 | 40% | - | + | - | 3:1 | PMF0 | 0.5 |
Al-Ali:Novartis: Honoraria, Research Funding. Lange:Novartis: Consultancy, Honoraria, Research Funding. Prashanth:Novartis: Employment. Niederwieser:Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Gentium: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Amgen: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal