Abstract
Background: Nilotinib elicits faster, deeper molecular responses than imatinib as frontline treatment for CML-CP and is also approved as second-line treatment for patients (pts) intolerant of or resistant to imatinib.However, the optimal management of pts with SoR to frontline imatinib treatment has not been determined. Here, we present final data from the Study to Evaluate Nilotinib in CML pts with SubOptimal Response (SENSOR, NCT0104387), in which adult pts with molecular SoR to frontline imatinib switched to nilotinib.
Methods: In this multicenter, open-label study, 45 pts received nilotinib 400 mg twice daily with 24 mo of follow-up. All pts had SoR per 2009 European LeukemiaNet criteria—complete cytogenetic response (CCyR) but no major molecular response (MMR; BCR-ABL1 ≤ 0.1% on the International Scale [IS]) after ≥ 18 mo of frontline imatinib. The primary endpoint was rate of MMR at 12 mo after switch to nilotinib. BCR-ABL1IS transcript levels using ABL1as a control gene were centrally evaluated monthly (mo 1-3), then every 3 mo. Durable MMR at 24 mo was defined as MMR at both 12 and 24 mo with no intermediate loss of MMR. MR4 and MR4.5 were defined as BCR-ABL1IS ≤ 0.01% and ≤ 0.0032%, respectively. Mutation analyses by direct sequencing were performed at baseline (BL) and at end of study for all pts. Retrospective mutational analyses were performed even in pts who did not have any mutations identified at BL or at the end of study visit.
Results: Pts were enrolled from Dec 2009 to Feb 2012; 39 pts completed the study and 6 discontinued. Reasons for discontinuation were adverse events (AEs; n = 3), disease progression (n = 1), and withdrawn consent (n = 2). Median dose intensity of nilotinib was 748.9 mg /day (range, 183-799 mg) for a median duration of exposure of 22.1 mo (range, 0.1-22.7 mo).
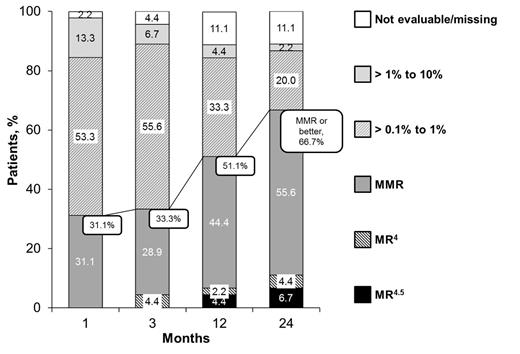
The primary endpoint was met; the rate of MMR at 12 mo was 51.1% (the expected value was 40%). Median time to first MMR among pts who achieved MMR (N = 23) was 2.28 mo, and 51.1% of pts had durable MMR. The proportion of pts achieving MMR as well as deeper molecular responses (MR4, MR4.5) increased over time (Figure). Rates of MMR, MR4, and MR4.5 were 66.7%, 11.1%, and 6.7%, respectively, at 24 mo. By 24 mo, the cumulative rates of MMR, MR4, and MR4.5 were 75.6%, 13.3%, and 6.7%, respectively. Overall, BCR-ABL1 levels decreased during treatment. The median BCR-ABL1IS ratio was 0.24% (range, 0.1%-3.5%) at BL (n = 45) and 0.06% (range, ≤ 0.0032%-2.3%) at 24 mo (n = 40). The slope of BCR-ABL decline was steepest in the first 3 mo after switch to nilotinib. Univariate and multivariate analyses of factors associated with molecular responses at 24 mo will also be presented.
Among 30 pts with MMR at 24 mo, 4 pts (13.3%) had BCR-ABL1 mutations at BL, and 17 pts (56.7%) had newly detected (post-BL) mutations (Table). One pt with a newly detected T315I mutation never achieved MMR, progressed to blast crisis after 5.4 mo, and died at 9.4 mo. No other pt progressed or died.
The most common any-grade AEs were hyperbilirubinemia (53.3%), nasopharyngitis (46.7%), and headache (37.8%). Elevated lipase level (20.0%) and hypophosphatemia (15.6%) were the most common grade 3/4 AEs.
Conclusions: Switching from imatinib to nilotinib resulted in 66.7% of pts achieving MMR at 24 mo and a cumulative incidence of MMR of 75.6% by 24 mo. Some pts achieved even deeper molecular responses. These responses were achieved regardless of BL mutation status and new mutations/splicing abnormalities detected on treatment, except for T315I. Further study is required to determine the impact of the mutations on the efficacy of nilotinib. The safety profile of nilotinib was consistent with prior studies.
MMR at 24 mo by BCR-ABL1 Mutation Status (Assay Method: Direct Sequencing)
| . | With MMR n = 30 . | Without MMR n = 15 . | ||
|---|---|---|---|---|
| BL n (%) . | Post-BL n (%) . | BL n (%) . | Post-BL n (%) . | |
| No mutation | 26 (86.7) | 13 (43.3) | 15 (100) | 6 (40.0) |
| Any mutation | 4 (13.3) | 17 (56.7) | 0 | 9 (60.0) |
| Insensitive | ||||
| T315I | 0 | 0 | 0 | 1 |
| E255K | 1 | 0 | 0 | 0 |
| Others (occurring in > 2 pts) | ||||
| Exon 8/9 35 base pair insertion | 1 | 9 | 0 | 9 |
| Exon 7 deletion | 1 | 4 | 0 | 3 |
| . | With MMR n = 30 . | Without MMR n = 15 . | ||
|---|---|---|---|---|
| BL n (%) . | Post-BL n (%) . | BL n (%) . | Post-BL n (%) . | |
| No mutation | 26 (86.7) | 13 (43.3) | 15 (100) | 6 (40.0) |
| Any mutation | 4 (13.3) | 17 (56.7) | 0 | 9 (60.0) |
| Insensitive | ||||
| T315I | 0 | 0 | 0 | 1 |
| E255K | 1 | 0 | 0 | 0 |
| Others (occurring in > 2 pts) | ||||
| Exon 8/9 35 base pair insertion | 1 | 9 | 0 | 9 |
| Exon 7 deletion | 1 | 4 | 0 | 3 |
Yamamoto:Novartis Pharma K.K.: Honoraria, Research Funding; Pfizer Inc.: Research Funding, Speakers Bureau, support with manuscript preparation, support with manuscript preparation Other; ARIAD Pharmaceuticals, Inc: Research Funding. Taniwaki:Novartis: Honoraria, Research Funding. Kimura:Novartis: Research Funding, Speakers Bureau; BMS: Speakers Bureau. Ohyashiki:Novartis Pharma K.K.: Honoraria, Research Funding, Speakers Bureau. Kawaguchi:Novartis Pharma: Honoraria. Matsumura:Novartis Pharma KK: Honoraria, Research Funding; Bristol-Myers Squibb Company: Honoraria. Hino:Novartis Pharma: Research Funding. Kondo:Novartis Pharma K.K.: Employment. Aoki:Novartis Pharma K.K.: Employment. Okada:Novartis: Employment. Yanada:Novartis: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal