Abstract
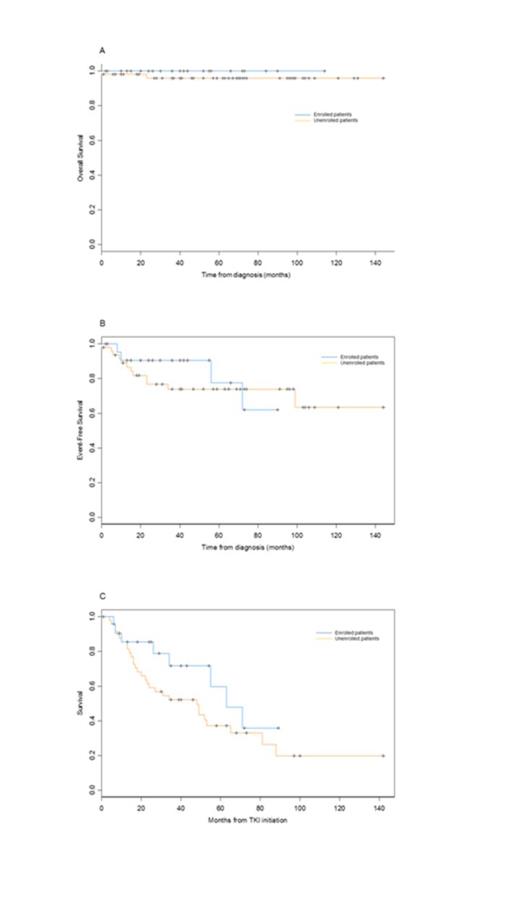
Chronic myeloid leukemia (CML) is a myeloproliferative disorder with a median age of approximately 60 years (Hoglund M 2013). However, little is known about outcomes in CML in adolescents and young adults. In the literature there are few reports involving only patients enrolled in trials aged from 15 to 30 years (Cortes J 2012, Kalmanti L 2013). We report here the characteristics and outcomes in 78 unselected adolescents and young adults ranging from 18 to 25 years with newly diagnosed CML in chronic (n=73) or in accelerated phase (n=5) in the tyrosine kinase inhibitor (TKI) era from 13 Fi-LMC centers being in possession with local databases. The median follow-up is 56 months (0-144) after diagnosis. Sokal scores were low in 41 (56%) patients, intermediate in 10 (13%), and high in 13 (17%) and unknown in 9 (14%) patients. Five patients had a CCA/Ph+ but were in CP cytologically, at diagnosis. Initial TKI were imatinib alone (n=55) or in combination with IFN (n=3), nilotinib alone (n=5) or in combination (n=1), dasatinib alone (n=10) or in combination (n=1) or ponatinib (n=1). One patient died before treatment initiation from brain hemorrhage, and initial treatment is unknown in one patient. Only 38/76 (50%) of patients remained under TKI first-line at latest follow-up. The reasons of first-line discontinuation were blast crisis (n=3); according to ELN criteria, cytogenetic failures (n=10), molecular failures (n=5), molecular warnings (n=8), mutation (n=1); intolerance (n=6), FDA notification in EPIC study (n=1). The second-line therapies were imatinib (n=2), IFN in combination with aracytine (n=1), nilotinib (n=13), dasatinib (n=13), high-dose chemotherapy alone (n=1) or followed by allogeneic bone marrow transplantation (n=4). 13 patients discontinued their second line TKI for blastic transformations (n=2), cytogenetic failures (n=4), molecular failures (n=2), molecular warning (n=1), mutation (n=1) and intolerance (n=3). The third-line therapies were imatinib (n=1), nilotinib (n=2), dasatinib (n=5), ponatinib (n=1), chemotherapy alone (n=1), allo-transplantation (n=1). Only 25/78 (32%) of patients were included in a trial. Only one patient experimented a 4th line of TKI (ponatinib). We compared characteristics and outcome of the 2 groups of patients, enrolled in first line versus unenrolled (Table 1). There were significantly more men included than women. Accelerated phases and CCA/Ph+ were observed only in the unenrolled group. The overall survival is shown in Figure 1A. Blastic transformation, failure of TKI defined as ELN 2013 recommendations and death were used to calculate the EFS curve (Figure 1B). Finally, we designed a curve representing the probability to remain under first-line TKI: 2nd line TKI-Free Survival (Figure 1C). A complete analysis comparing characteristics and outcome between the 2 groups of patients, will be available for ASH presentation.
Characteristics of patients
| . | Total patients n=78 . | Enrolled patients n=24 . | Unenrolled patients n=54 . | P value . |
|---|---|---|---|---|
| Ratio H/F (n) | 50/28 | 18/5 | 22/32 | 0.0025 |
| Median age (years) | 22 | 23 | 22 | NS |
| CCA/Ph+ (n) | 5 | 0 | 5 | - |
| Phase (C/A) (n) | 73/5 | 24/0 | 49/5 | NS |
| Sokal (L/I/H/U) (n) | 41/10/13/9 | 16/2/5/1 | 25/8/8/8 | NS |
| Median FU (months) (Range) | 56 (0-144) | 41 (2-114) | 63 (0-144) | - |
| Interval from D to 1st line TKI (days) | 27 | 29 | 24 | - |
| 1st line TKI (I/N/D/P/NA)(n) | 58/6/11/1/2 | 9/4/10/1/0 | 49/2/1/0/2 | NS |
| Median 1st line TKI duration (months) | 27 | 26 | 28 | - |
| Discontinuations (n) | 38 | 8 | 30 | 0.069 |
| Discontinuation reasons (n) Blastic phase Cytogenetic failure Molecular failure Molecular warning Intolerance Pregnancy Sustained CMR FDA notification Mutation | 3 10 5 8 6 2 2 1 1 | 0 3 3 0 0 0 1 1 0 | 3 7 2 8 6 2 1 0 1 | |
| TKI line Number (1/2/3/4)(n) | 38/19/10/1 | 17/5/2/0 | 24/12/8/1 | - |
| . | Total patients n=78 . | Enrolled patients n=24 . | Unenrolled patients n=54 . | P value . |
|---|---|---|---|---|
| Ratio H/F (n) | 50/28 | 18/5 | 22/32 | 0.0025 |
| Median age (years) | 22 | 23 | 22 | NS |
| CCA/Ph+ (n) | 5 | 0 | 5 | - |
| Phase (C/A) (n) | 73/5 | 24/0 | 49/5 | NS |
| Sokal (L/I/H/U) (n) | 41/10/13/9 | 16/2/5/1 | 25/8/8/8 | NS |
| Median FU (months) (Range) | 56 (0-144) | 41 (2-114) | 63 (0-144) | - |
| Interval from D to 1st line TKI (days) | 27 | 29 | 24 | - |
| 1st line TKI (I/N/D/P/NA)(n) | 58/6/11/1/2 | 9/4/10/1/0 | 49/2/1/0/2 | NS |
| Median 1st line TKI duration (months) | 27 | 26 | 28 | - |
| Discontinuations (n) | 38 | 8 | 30 | 0.069 |
| Discontinuation reasons (n) Blastic phase Cytogenetic failure Molecular failure Molecular warning Intolerance Pregnancy Sustained CMR FDA notification Mutation | 3 10 5 8 6 2 2 1 1 | 0 3 3 0 0 0 1 1 0 | 3 7 2 8 6 2 1 0 1 | |
| TKI line Number (1/2/3/4)(n) | 38/19/10/1 | 17/5/2/0 | 24/12/8/1 | - |
Abbreviations: U unknown; FU follow up; D diagnosis; I imatinib; N Nilotinib; D dasatinib; P Ponatinib; TKI Tyrosine Kinase Inhibitor
(A) overall survival from diagnosis of enrolled and unenrolled patients. (B) EFS of enrolled and unenrolled patients. Patients in AP were excluded. (C) 2nd line TKI-Free Survival of enrolled and unenrolled patients corresponding to the probability to remain under first-line TKI.
(A) overall survival from diagnosis of enrolled and unenrolled patients. (B) EFS of enrolled and unenrolled patients. Patients in AP were excluded. (C) 2nd line TKI-Free Survival of enrolled and unenrolled patients corresponding to the probability to remain under first-line TKI.
Nicolini:Novartis: Consultancy. Gardembas:BMS: Honoraria. Etienne:Novartis, BMS, Pfizer, Ariad: Honoraria. Guerci-Bresler:Novartis, BMS, Pfizer: Honoraria. Roy:Novartis: Honoraria; BMS: Honoraria. Legros:Novartis, BMS: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal