Abstract
Background: Constitutively activated PI3K/AKT/mTOR pathway plays a critical role in the proliferation and survival of cancer cells, through the expression of numerous pro-survival and proliferative genes. Specific inhibitors of the various isoforms of PI3K have shown promising activity in the treatment of indolent B-cell lymphoma. However, they have not shown similar activity in aggressive lymphoma, potentially because the expression of many PI3K/AKT/mTOR dependent pro-survival genes may be activated by alternative signals, for example, NF-kappaB (NF-kB). While specific inhibitors of NF-kB are currently not available in the clinic, proteasome inhibitors are well known to inhibit NF-kB, among its pleiotropic mechanisms of action. We hypothesize that if cancer cell survival is dependent on a complex protein network involving PI3K and NF-kB, then complimentary inhibition of PI3K and the proteasome will become highly synergistic. Our results provide strong support for the hypothesis, by demonstrating that TGR-1202, a PI3Kdelta inhibitor, and carfilzomib, a proteasome inhibitor, are unique among the combinations of PI3K and proteasome inhibitors in potently suppressing the mTORC1 complex and NF-kB, and are highly effective in models of B- and T-cell lymphoma.
Methods: Cytotoxicity of PI3K and proteasome inhibitors was studied in a panel of B and T-cell lymphoma cell lines. Growth inhibition was determined using Cell Titer Glo that measures ATP produced by live and proliferating cells. Cell death was determined by flow cytometry, using the probes Annexin V and propidium iodide. Drug: drug synergy was determined by calculating the combination index (CI) and relative risk ratio (RRR). Values below 1 indicate synergy, with smaller RRR and CI values indicating higher levels of synergy. The mechanistic basis of the synergy is determined through in-depth interrogation of the PI3K/AKT/mTOR pathway and its downstream targets and interacting pathways.
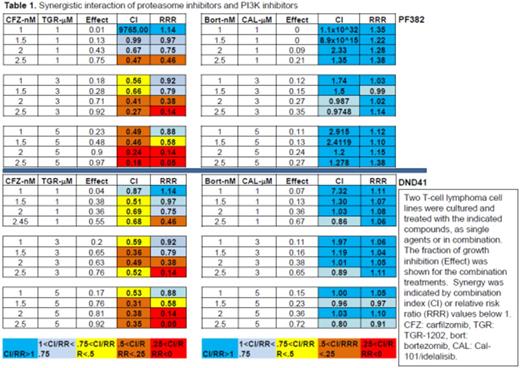
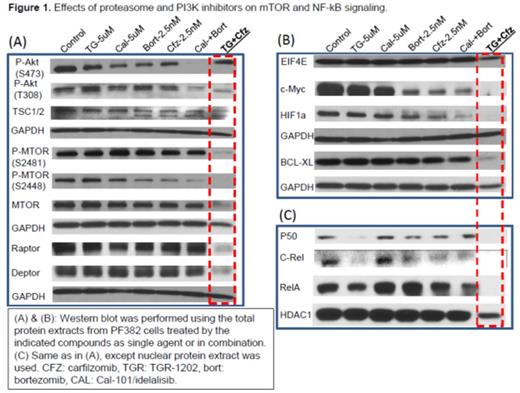
Results: We used a high-throughput platform to screen the cytotoxicity of TGR-1202, idelalisib/CAL-101, bortezomib, and carfilzomib, in a panoply of B- and T-cell lymphoma cell lines. We found that TGR-1202 and idelalisib as single agent caused only minimal to mild cytotoxicity in lymphoma cells. Bortezomib and carfilzomib as single agent caused dose dependent inhibition of cell growth. Surprisingly, when TGR-1202 or Cal-101 were combined with bortezomib or carfilzomib in a 2x2 design, the combination of TGR-1202 and carfilzomib was consistently the most synergistic, while the other 3 combinations were either much less or not at all synergistic. For example, Table 1 clearly demonstrated that TGR-1202 and carfilzomib demonstrated much higher inhibitory effect and stronger synergy than Cal-101 and bortezomib in two T-ALL cell lines. We have systematically interrogated the effects of these drug combinations on the PI3K/AKT/mTOR pathway, and found that the combination of TGR-1202 and carfilzomib was able to uniquely and potently inhibit components of the mTORC1 complex, causing inhibition of mTORC1 dependent signals such as phosphorylated 4E-BP1, leading to markedly reduced expression of critical genes such as c-myc (Figure 1). Furthermore, the combination of TGR-1202 and carfilzomib uniquely and potently inhibited activation of NF-kB, resulting in markedly suppressed expression of Bcl-xL. The complementary inhibition of the PI3K/AKT/mTOR and NF-kB pathways resulted in robust growth inhibition and apoptosis (Figure 1), and warrants further clinical evaluation for aggressive B- and T-cell lymphoma.
Sportelli:TG Therapeutics: Employment, Equity Ownership. Miskin:TG Therapeutics, Inc.: Employment, Equity Ownership. Viswanadha:Incozen: Employment.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal