Abstract
Background: Marginal zone lymphomas (MZLs) account for 10% to 17% of all non-Hodgkin lymphomas (NHLs). Eradication of Helicobacter pylori (HP) is considered the initial therapy of choice for localized HP-positive gastric MALT and radiation is commonly used for other localized MALT or nodal MZL. While radiotherapy in localized MALT and nodal MZLs frequently results in long term local control and prolonged progression-free survival (PFS), it is not always feasible. Consensus guidelines on the best upfront therapy for patients with non-gastric MALT and disseminated extranodal MZLs are absent. Considering the excellent local control of MZLs with radiotherapy, targeted delivery of radiation by radioimmunotherapy may be the preferential approach for patients with untreated disseminated MZL. Based on this premise, we designed a single center Phase II clinical trial to examine 90Y Ibritumomab in the upfront treatment of patients with Marginal Zone Lymphoma.
Methods: Eligible patients were 18 years and older with a diagnosis of untreated, histologically proven CD20-positive marginal zone lymphoma of all stages, including extranodal and nodal MZL variants. Patients with HP-negative and patients with HP-positive gastric MALT lymphoma who did not respond to antibiotic therapy were also eligible. Additional inclusion criteria were: measurable disease (at least one lymph node or tumor mass 2 cm in any axis), Eastern Cooperative Oncology (ECOG) performance status of 0, 1 or 2 and adequate bone marrow (neutrophils >1.5x109/L; platelets >100x109/L), hepatic and renal functions. Treatment consisted of intravenous rituximab 250 mg/m² on day 1 with appropriate premedications. On Day 8, patients received a second dose of rituximab 250 mg/m² prior to 0.4 mCi/kg (14.8 MBq/kg) of 90Y ibritumomab tiuxetan administered as a 10-minute intravenous push. Response assessment was made at week 12 post therapy by CT scan and physical examination. Patients underwent follow up with regular clinical reviews and CT scans of the neck, chest, abdomen, and pelvis every 3 months for the first 2 years and then every 6 months for an additional 3 years.
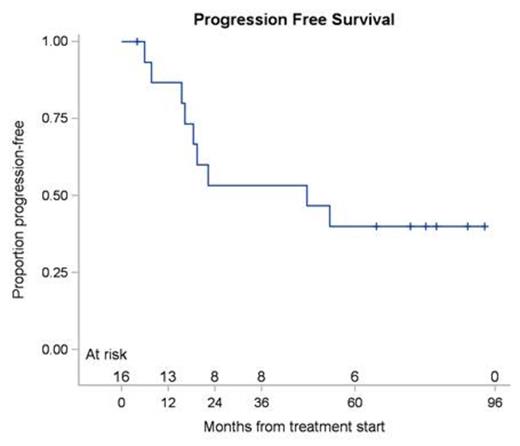
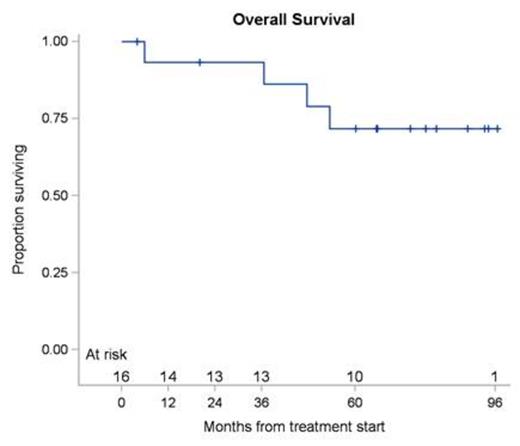
Results: Between June 2006 and January 2014, 16 patients were enrolled. The median age was 62 years with a range of 37 to 84 years. The ORR at week 12 post treatment was 87.5% (14 of 16 patients) with a 90% confidence interval of 65.6% to 97.7%. The responses comprised of CR in 8 (50%), CRu in 1 (6%) and PR in 5 (31%) patients. A total of 2 patients (13%) were defined as having stable disease at week 12 restaging. With a median follow up of 65.6 months (range 4.0-96.5), the median PFS was 47.6 months (range 4.0-93.3) for all patients (Figure 1A), with median PFS of 47.6 months (range 4.0-74.2) for patients achieving CR. Of the 16 treated patients, 4 died (25%). None of the deaths was directly attributed to MZL and 2 patients died while being in continuous CR. Median OS by Kaplan-Meier method for all the patients was not attained (Figure 1B). The 5-year PFS was 40% (90% CI: 19.9% - 59.5%) and 5-year OS was 71.8% (90% CI: 46.8% - 86.5%). The principal toxicity was hematological. Reversible grade 3 or 4 neutropenia occurred in 8 of 16 (50%) of patients, median duration 21 days (range 9-69 days). Reversible grade 3 or 4 thrombocytopenia was observed in 5 of 16 (31.3%) patients with a median duration of 28 days (range 13-42 days). Grade 4 anemia occurred in one (6.3%) patient and lasted for 15 days. Later adverse non-hematological events were typically mild and self-limiting.
Conclusion: Our phase-II trial suggests that one single injection of 90Y ibritumomab tiuxetan may be a valid therapeutic option for front-line therapy of patients with MALT lymphoma, producing response rates similar to those achieved with immunochemotherapy treatment regimens. Further evaluation of this therapeutic approach in a prospective, preferentially randomized, multi-institutional clinical trial is needed.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal