Abstract
Burkitt lymphoma (BL) is a rare disease that account for 2% of all cases of non-Hodgkin lymphoma in adults. Several treatments have been published so far with improving response rates obtained after augmentation with Rituximab of the original protocols. We have previously published the results of a short and intensive chemotherapy regimen translated from the pediatric patients to adults. Here we update and extend our previous results to a cohort of 51 patients with confirmed diagnosis of BL.
From December 1991 to January 2014, we enrolled 51 patients with Burkitt lymphoma. The original regimen was applied to the first 22 patients who were treated at the Fondazione IRCCS Istituto Nazionale dei Tumori in Milan. The treatment consisted of and induction phase comprising CTX (500 mg/mq, day 1-2) and VCR (1,4 mg/mq day 1); MTX (150 mg/kg day 8), VP-16 (250 mg/mq q 12 hs, day 14); MTX (250 mg/kg day 21), ADM (50 mg/mq, day 28), VCR (1,4 mg/mq, day 35), and a consolidation phase with HD ARA-C (1000 mg/mq continuos infusion for 4 days with a daily extra dose of 750 mg/mq) plus CDDP (20 mg/mq 4 day continuos infusion). Weekly intratechal chemotherapy for 6 doses was administered. Patients not achieving complete remission (CR) before consolidation, were shifted to sequential high-dose chemotherapy (HDS) comprising HD-CTX and stem cell harvest, HD-ARAC, Dexa-BEAM and BEAM followed by infusion of autologous stem cell transplant (ASCT). Rituximab was included in R-HDS only for in-vivo purging of collected stem cells. An updated version of the protocol was introduced from year 2003 and was applied to a set of 39 patients enrolled by the Gruppo Italiano Terapie Innovative nei Linfomi (GITIL) in four different centers. The new regimen was augmented with rituximab both in the induction (on day 15, day 29, day 36) and after consolidation (two weekly administrations) phases. Also for R-HDS salvage regimen, more rituximab infusions were added to the original schema for a total of 6 administrations. Patients characteristics were as follow: M/F 33/18, median age 46 years (range 18-90 years), stage I-II/III-IV 22/29, IPI H or HI 24 patients, bulky disease 20 patients, CNS involvement in 4 subjects, high LDH levels in 25 patients, ECOG performance status (PS) 0-1 vs 2-3 33/18, BM involvement in 7 subjects.
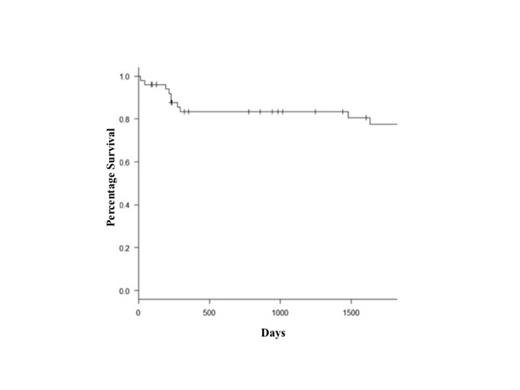
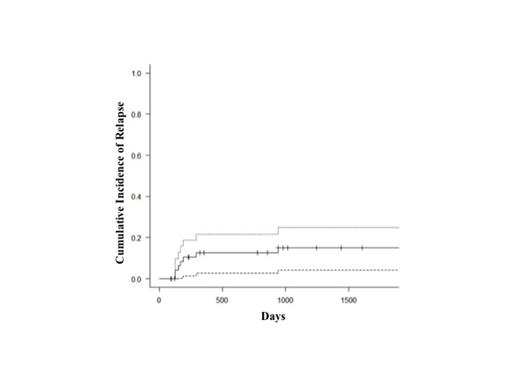
Of 49 patients evaluable for response (2 died of early regimen-related toxicity), CR was obtained after consolidation in 80% of the subjects and was improved to 94% by the R-HDS salvage regimen. In details, of the 4 subjects with PD and the 3 with PR after the induction phase, only one and all three were rescued by R-HDS treatment, respectively. Three patients relapsed after the short-intensive regimen: of them one was saved with R-HDS. With a median follow-up of 1994 days, Kaplan-meier estimates of 5-year OS and PFS were 78% (Figure 1) and 69% respectively, and cumulative incidence of relapse, with Gray test, was 15% (95% CI, 6.5-27; Figure 2). We did not observe any difference in terms OS (p=0.6) and DFS (p=0.7) between patients treated before 2003 by a single center or after 2003 when other hospitals participated to the study.
In this study we extended our previous finding and confirmed that the short intensive chemotherapy program achieves high percentages of complete response in patients with BL, while those with primary refractory disease can effectively be saved by the R-HDS regimen. In addition, we demonstrated that our program is easily exportable to other hospitals with reproducible results in a multicenter setting.
5-year OS of patients with Burkitt lymphoma treated with a short-intensive chemotherapy regimen.
5-year OS of patients with Burkitt lymphoma treated with a short-intensive chemotherapy regimen.
5-year Cumulative Incidence of relapse (with 95% confidential interval) of patients with Burkitt lymphoma treated with a short-intensive chemotherapy regimen
5-year Cumulative Incidence of relapse (with 95% confidential interval) of patients with Burkitt lymphoma treated with a short-intensive chemotherapy regimen
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal