Abstract
Background. CD5+ diffuse large B-cell lymphomas (DLBCLs) are an uncommon subgroup of DLBCLs representing 5-10% of de novo DLBCLs. Compared with CD5- DLBCLs, de novo CD5+ DLBCLs are associated with a more aggressive clinical course with higher international prognostic index (IPI), more frequent extranodal disease burden including central nervous system (CNS) involvement, and a significantly poorer overall prognosis with conventional anthracycline-based chemotherapy with or without rituximab (Miyazaki K et al. Ann Oncol 2011). Historically, most of these cases belong to the activated B cell (ABC)-type of DLBCLs (Jain P et al., Am J Hematol 2013). The vast majority of the published reports on CD5+ DLBCL are from Asia with limited data on outcomes in US or European patients (pts) in the modern era utilizing rituximab based therapy with no standard guidelines available for the management of this high risk disease.
Methods. We reviewed pts with newly diagnosed de novo CD5+ DLBCL treated at the Ohio State University (OSU) from August 2000 to July 2014. Pts did not have an antecedent history of CLL or MCL and diagnosis was confirmed by centralized hematopathology review at OSU. We examined pts' characteristics, treatment response, and survival. Response to treatment was determined by PET/CT. Progression-free survival (PFS) was calculated as the time from diagnosis to the time of progression and/or death, and overall survival (OS) was calculated as time from diagnosis to the time of death. PFS and OS were summarized using Kaplan-Meier methods.
Results. Of the 16 CD5+ DLBCL, 11 (70%) were male, and the median age at diagnosis was 58 (range 44-67). Eight pts had CD5+ DLBCL ABC-type, 4 had germinal center (GCB)-type and 4 were non-classifiable by Hans criteria. At diagnosis, 11 pts were stage III/IV; 6 pts had bone marrow involvement, 2 had CNS involvement; and 2 pts had bulky disease (≥10cm). IPI was ≥ 3 in 4 pts (unable to calculate in 6 pts due to unknown LDH) and 2 pts had B symptoms. The median Ki67 was 75% (range 50-95%), EBER was negative in all pts; 3 of 13 evaluable pts were positive for MYC gene rearrangement, and 0 pts had an IGH/BCL2 translocation. Therapies at diagnosis included R-CHOP (12 pts), R-EPOCH (2 pts), R-CHOP with 3 g/m2 of methotrexate (1 pt), and hyperfractionated cytoxan (1 patient). In addition, 2 pts received IT methotrexate prophylaxis and 5 pts received involved field radiation therapy to residual disease sites post frontline chemotherapy. None of the pts underwent autologous stem cell transplant (SCT) in first remission. Thirteen pts (81%) achieved a complete remission (CR) with frontline treatment. Three patients had primary refractory disease, 2 rapidly progressed and died within 1 month from diagnosis.
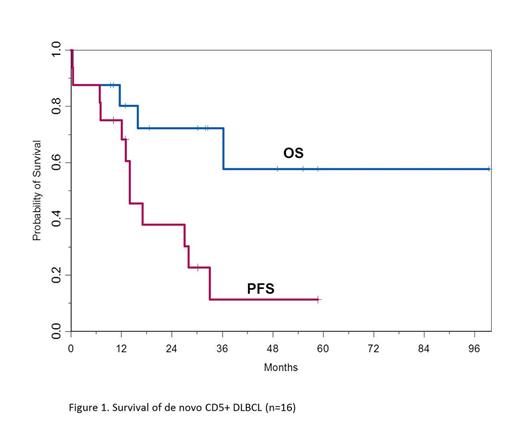
Of the 13 pts in CR, 4 (31%) remain in remission with a median follow up of 28 months (range 10-61). Nine pts in CR relapsed (69%), including 6 pts who proceeded to salvage therapy and autologous SCT, 1 pt who received both autologous and allogeneic SCT, 1 pt who underwent allogeneic SCT, and 1 pt who received multiple lines of salvage chemotherapy with no transplant and eventually died. Five of the 6 pts (84%) that underwent autologous SCT relapsed and both pts that underwent allogeneic SCT relapsed. Ten pts (63%) received ≥ 3 lines of chemotherapy. Of the 16 pts, 5 (31%) have died from disease progression. At a median follow up of 32 months (range 9.4 to 99.4), the 2 year PFS and OS for all pts were 38% and 72%, respectively (Figure 1). The median time to progression (TTP) from frontline therapy was 14 months (95% CI 7-28 months). The median TTP from second line therapy in 10 pts was 17 months (95% CI 7-33 months).
Conclusions. To the best of our knowledge,this is the first series of CD5+ DLBCL from Western countries in the PET/rituximab era reported to date. With the recognized limitation of a small sample size and retrospective analysis, our current study confirms that CD5+ DLBCL pts have a poor prognosis which appears to be inferior to CD5- DLBCL despite a multimodality approach. Interestingly, autologous as well as allogeneic SCT at relapse do not seem to improve outcome, raising the question if there is even a role for transplant in these pts outside the context of a clinical trial. Similar to the initial reports about the poor prognosis of MYC translocation and DLBCL (Savage KJ et al. Blood 2009), we suggest that CD5 positivity is a particularly poor prognostic marker and should be used for risk stratification in DLBCL clinical trials.
Jones:Pharmacyclics: Consultancy, Research Funding. Blum:Janssen, Pharmacyclics: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal