Abstract
Diffuse large B-cell lymphoma (DLBCL) is a heterogeneous disease both biologically and clinically. Although a number of genetic alterations have been associated with the pathogenesis of DLBCL, survival association of most of the identified mutations remains to be shown. Here we have used exome and RNA sequencing (RNAseq) data to identify novel genetic events associated with treatment outcome after chemoimmunotherapy.
Initial screen was performed with eight primary tumor samples and matched normal DNA using exome sequencing. The patients had clinically high risk DLBCL, and were treated in a Nordic phase II protocol with dose dense chemoimmunotherapy and CNS prophylaxis. After stringent filtering (VarScan2 p < 0.05), and removal of known germline variants, 967 somatic point mutations and indels in 561 different genes were identified. Of these, 436 were non-synonymous, and 27 were detected in more than one patient. These included both novel and several previously described DLBCL associated mutations, such as MYD88, CD79B, B2M and PTEN.
To correlate the identified mutations with survival, we used RNAseq and survival data of 92 DLBCL samples from the Cancer Genome Characterization Initiative (CGCI) repository. The mutational status of the 436 genes identified in our screen was assessed with Bambino, which confirmed 405 of these genes also to be mutated in the CGCI set. Kaplan-Meier survival estimates per gene with at least one single nucleotide variant (SNV; with criteria of minimum 3 reads and 10% coverage and known polymorphisms filtered out) revealed 17 genes that were associated with overall survival (OS, p<0.05, minimum 10 samples mutated).
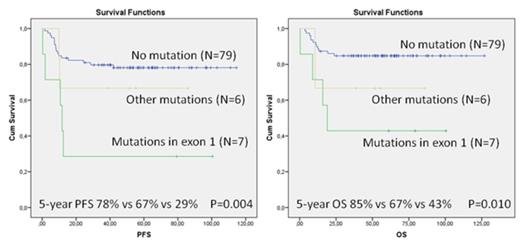
We highlight mutations affecting Deltex1 (DTX1), which functions as an E3 ubiquitin ligase and targets Notch1 intracellular domain for degradation. In the study cohort, 14% of the patients had a non-synonymous DTX1 mutation. Mutations were equally distributed between GCB and ABC DLBCLs and low (0-2) and high (3-5) International Prognostic Index (IPI) risk groups. According to Kaplan-Meier analysis, the 5-year PFS rate for the patients with DTX1 mutations was significantly worse as compared to ones without mutations (46% vs 78%, p=0.010). The corresponding OS values were 54% and 85% (p=0.006). In multivariate analysis with IPI, DTX1 mutation status remained as an independent prognostic factor for both PFS (RR, 2.83; 95% CI, 1.17-6.85, p=0.021) and OS (RR, 3.51; 95% CI, 1.41-8.71, p=0.007). Mutational status was also found to correlate with the expression levels; the patients with mutations had lower DTX1 mRNA levels (p=0.006). When DTX1 mutations were analyzed according to different functional domains, 11 out of 16 (65%) were located in the first exon encoding the WWE 1 domain and a binding site for intracellular domain of Notch1. Interestingly, the patients with mutations in exon 1 had a significantly worse survival in comparison to other patients (Figure 1).
Taken together, the results demonstrate that DTX1 mutations in DLBCL are an independent predictor of survival after chemoimmunotherapy, and mutations enriched in exon 1 identify cases with dismal prognosis.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal