Abstract
Total-body irradiation is frequently used as a conditioning treatment for hematopoietic stem cell transplantation. Although previous studies have demonstrated that irradiation induces apoptosis and senescence in hematopoietic stem/progenitor cells (HSPCs), its effect on the functional characteristics of human bone marrow mesenchymal stromal/stem cells (BM-MSCs) is largely unknown.
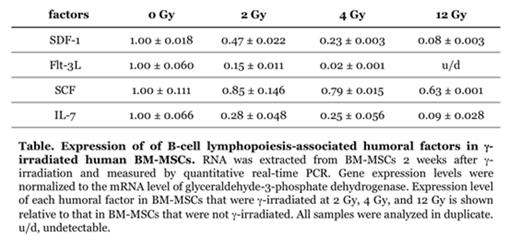
Human BM-MSCs were isolated from BM samples according to our previously published method (Stem Cells 32:2245, 2014). BM samples were purchased from AllCells (Emeryville, CA). For the irradiation experiments, BM-MSCs were g-irradiated (Cesium-137) with various doses ranged from 2 to 12 Gy by a Gammmacell Irradiator (Best Theratronics Ltd, Ontario, Canada). We first examined the expansion of g-irradiated human BM-MSCs. When BM-MSCs (0.5 x 105) were cultured on a 10-cm culture dish in advanced-minimal essential medium (Invitrogen, Carlsbad, CA) supplemented with 5% fetal bovine serum (FBS, Invitrogen), the cells expanded rapidly, reached near confluence within 2 weeks, and the average number of cells on day 7 was 6.4 x 105. On the other hand, the number of BM-MSCs that were g-irradiated at 2 Gy, 4 Gy and 12 Gy on day 7 was low at 0.8 x 105, 0.3 x 105, and 0.2 x 105, respectively. The recovery of cell expansion was irradiation dose-dependent; the average number of cells on day 28 was 8.6 x 105 (2 Gy), 3.7 x 105 (4 Gy) and 0.3 x 105 (12 Gy). Next, hematopoiesis-supportive capabilities of g-irradiated human BM-MSCs were examined. Human CD34 positive HSPCs were co-cultured with g-irradiated BM-MSCs in StemSpan Serum-Free Expansion Medium (STEMCELL Technologies, Vancouver, Canada) supplemented with stem cell factor (SCF), Flt3-ligand (Flt3-L), thrombopoietin (TPO), and interleukin (IL)-3. After 10-day co-culture, the expansion of HSPCs was comparable among BM-MSCs with or without g-irradiation. The number of CD33 positive myeloid progenitor cells in the expanded cells was also comparable among BM-MSCs with or without g-irradiation. However, when human CD34 positive HSPCs were co-cultured with g-irradiated BM-MSCs in the complete medium supplemented with 10 ng/mL SCF and 5 ng/mL FLt3-L for 4 weeks, the generation of CD19 positive cells was impaired. The number of CD19 positive cells, which were generated in co-cultures of CD34 positive HSPCs (0.2 x 104) with BM-MSCs that were not g-irradiated, was 1.4 x 104, whereas those in co-cultures with BM-MSCs that were g-irradiated at 2 Gy, 4 Gy and 12 Gy were 0.09 x 104, 0.04 x 104, 0.05 x 104, respectively. With respect to the expression of B-cell lymphopoiesis-associated humoral factors in BM-MSCs, mRNA expression levels of CXCL12/SDF-1, Flt3-L, SCF and IL-7 were decreased in g-irradiated BM-MSCs. Especially, the expression of Flt3-L in BM-MSCs was reduced soon after irradiation exposure. Finally, we found that the osteogenic, adipogenic and chondrogenic differentiation capability of the g-irradiated BM-MSCs were dysregulated, as assessed by both the expression of lineage-specific molecular markers.
In conclusion, g-irradiation compromised expansion, differentiation and B-cell lymphopoiesis-supportive capabilities of human BM-MSCs in a dose-dependent manner. This study could provide new insights into the role of BM-MSCs in the pathogenesis of immunologic and non-immunologic complications after hematopoietic stem cell transplantation.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal