Abstract
BACKGROUND
Dabigatran etexilate has received its market authorization for various indications worldwide. It was developed to be used in fixed dose regimens without the need of regular monitoring. However, the perioperative management of dabigatran could require an assessment of the drug plasma levels to ensure a safe use of the product, especially in absence of specific antidotes. The EMA stated that dabigatran concentrations under 48 ng/mL should be reached before invasive intervention. The GIHP put the threshold at 30 ng/mL. Therefore, a specific laboratory assay, accurate in the low concentration range, easily available and performable 24h/7 is requested but, until now, all coagulation tests dedicated to the measurement of plasma dabigatran concentrations showed a lower limit of quantitation from 30 to 50 ng/mL. This limits their perioperative utility and the thrombin time (TT) is currently presented as an alternative due to its very high sensitivity to dabigatran.
This interesting approach has limitations because TT is affected by several analytical and biological variables that could lead to misinterpretations and expose the patient at riskier hemostatic conditions. In this study, we propose to investigate the performance of two coagulation tests (the Hemoclot Thrombin Inhibitors LOW (HTI LOW) (Hyphen BioMed) and the Ecarin Chromogenic Assay II (ECA-II) (Diagnostica Stago)) specifically developed to measure low plasma dabigatran concentrations and to compare their results with a reference LC-MS/MS. We also assessed the performance of the standard procedure of HTI and TT.
MATERIALS AND METHODS
Thirty-three plasma samples of patients treated with dabigatran etexilate for stroke prevention in non-valvular atrial fibrillation, were included in the study. Plasma samples were taken randomly and included after a first screening using the HTI to select plasma concentrations <200 ng/mL.
For HTI, tested plasma was diluted 1:8 in Owren-Koller® buffer. Fifty μl of tested plasma were mixed with 100 μl of normal pooled plasma and were incubated during 240 sec. One hundred μl of highly purified human thrombin pre-incubated at 37°C was then added to start the reaction. For HTI LOW, the dilution of the sample was reduced to 1:2 and specific calibrators at lower concentrations were used.
For ECA-II, tested plasma was diluted 1:5 in Owren-Koller® buffer. Fifty μl of tested plasma were mixed with 140 μl of prothrombin and then 70 µl of chromogenic substrate were added and incubated during 240 sec. Seventy μl of ecarin pre-incubated at 37°C were then added to start the reaction.
Thrombin time was performed using Thrombin Time® reagent 1.5NIH (Diagnostica Stago) and the limit of measurement was extended to 300 sec.
All of these procedures were performed on a STA-R Evolution® coagulometer (Diagnostica Stago).
RESULTS AND DISCUSSION
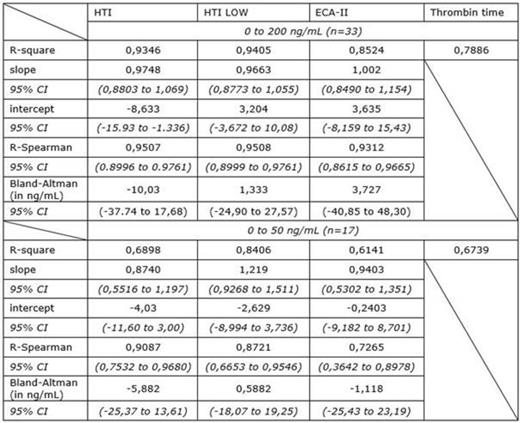
The plasma concentrations ranged from 0 to 200 ng/mL as provided by LC-MS/MS measurements. Among these samples, 17 were between 0 and 50 ng/mL. Linear correlation, Spearman correlation and Bland-Altman analyses versus LC-MS/MS are provided in Figure 1, for assays that express results in ng/mL, i.e. HTI, HTI LOW and ECA-II. For TT, the relation is described by linear correlation.
Our results show that HTI LOW performs better than HTI and ECA-II on the whole concentration range providing closer correlation and a lower systematic difference compared to LC-MS/MS. For concentrations below 50 ng/mL HTI LOW and ECA-II reveal similar systematic difference with higher 95% CI for ECA-II and perform better than HTI to estimate plasma concentrations. Thrombin time is less useful than dedicated assays to provide an estimation of the pharmacodynamics of dabigatran. For concentrations above 50 ng/mL it often exceeds the limit of measurement, i.e. 120 sec as defined by the manufacturer, and is influenced by the level of fibrinogen. However, it gives an acceptable correlation for concentration below 50 ng/mL. This may help the biologist to choose which test to use to avoid unnecessary costs and ensure the use of the more accurate dedicated assay to estimate the plasma concentrations. When TT is lower than 120 sec, HTI LOW or ECA-II should be preferred to HTI.
CONCLUSION
We recommend the use of specific coagulation assays to assess the dabigatran plasma concentrations before invasive procedure. Thrombin time may guide the biologist on the dedicated coagulation test to perform if he uses the HTI platform.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.
This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal