Abstract
Background:Dabigatran is approved for stroke prevention in atrial fibrillation (SPAF) in many countries. However, little is known about the effectiveness and safety of or the persistence with Dabigatran therapy in unselected patients in daily care.
Objectives: To evaluate the effectiveness, safety and discontinuation rates of dabigatran anticoagulation in SPAF in daily care.
Patients and methods:The prospective NOAC registry was initiated in November 2011. A network of more than 230 physicians in the district of Saxony, Germany, enroll up to 3000 patients in the registry, which are prospectively followed by the central registry office for up to 36 months. Persistence to dabigatran, rates of stroke/TIA/systemic embolism and of NMCR or major bleeding (ISTH definition) during or within 3 days after last intake of dabigatran were assessed using Kaplan-Meier time-to-first-event analysis.
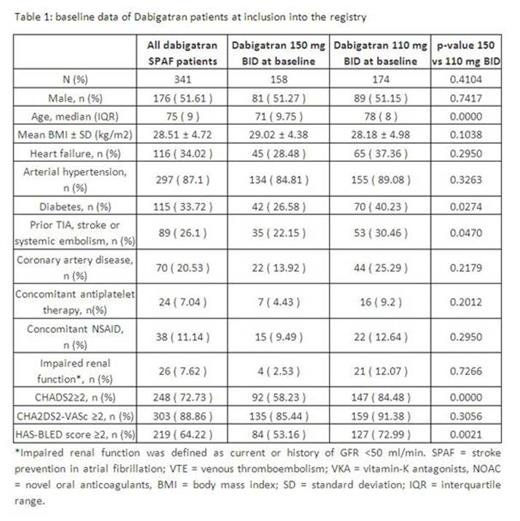
Results:Between November 2011 and February 2013, 341 SPAF patients were enrolled with dabigatran (133 with VKA pre-treatment and 208 newly anticoagulated patients). 158 received dabigatran 150 mg BID and 174 received 110 mg BID. Patients on 110 mg BID were significantly older (78 vs. 71 years), more often had diabetes, a history of stroke, a CHADS2 score ≥ 2 and HAS-BLED scores ≥2 (table 1).
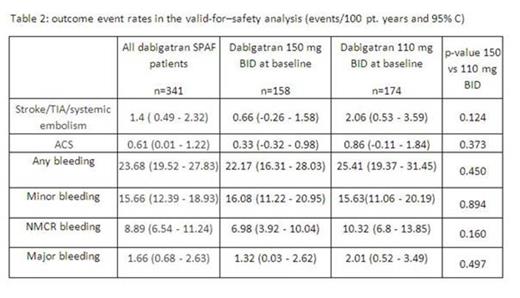
In the intention-to-treat analysis, stroke/TIA/systemic embolism occurred at a rate of 2.14/100 pt. years. In the valid-for safety analysis (all events during or within 3 days after last intake of dabigatran) stroke/TIA/systemic embolism occurred at a rate of 1.4/100 pt. years, which was numerically lower for patients receiving 150 compared to 110 mg BID (0.7 vs 2.1/100 pt. years; table 2).
Major bleeding occurred at a rate of 1.7/100 pt. years and numerically more often in patients receiving 110 instead of 150 mg BID (2.0 vs 1.3/100 pt. years). Similarly, rates of NMCR bleeding (8.9/100 pt. years) were numerically higher in the 110 mg BI cohort (10.3/100 pt. years) compared to the 150 mg BID cohort (7.0/100 pt. years).
Treatment discontinuation occurred in a total of 124 patients during follow up, which in a Kaplan-Meier analysis translated into a discontinuation rate of 23.8/100 pt. years. Documented reasons for treatment discontinuation were side effects (40/124; 32.3%), no longer indicated (13/124; 10.5%), worsening of renal function (12/124; 9.7%), bleeding complications (11/124; 8.9%), costs (4/124; 3.2%), inconvenience (13/124; 10.5%); others (31/124; 25%).
Conclusion:In unselected patients in daily care, dabigatran is effective and safe with low rates of cardiovascular or major bleeding events. However, within 12 month, about 24.6% (84/341) of patients are switched to other anticoagulants.
Werth:Bayer: Honoraria. Köhler:Bayer: Honoraria. Beyer-Westendorf:Boehringer Ingelheim: Honoraria, Research Funding; Pfizer: Honoraria, Research Funding; Bayer: Honoraria, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.
This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal