Abstract
This novel plasma derived, double viral attenuated VWF/FVIII concentrate with a ratio of 1:1 between both coagulant proteins (Wilate) was utilized in a pivotal clinical study conducted to further investigate its clinical efficacy and safety in VWD patients requiring surgery.
This was a prospective, open-label, uncontrolled, multi-center, phase III clinical study. The primary objective was to evaluate the overall hemostatic efficacy of Wilate in preventing excessive intra- and post-operative bleeding in pediatric and adult patients with VWD. Forty–one patients, 20 major and 10 with Type 3 VWD, were planned to be enrolled to document 41 surgical procedures. During the planned interim analysis after 30 procedures, a pre-specified success rate for study termination was reached and the study was stopped early as determined by an independent data and safety monitoring board (IDMC). In vivo recovery (IVR) was investigated in all patients at the beginning of the study for pre- and post-surgical dosing. Hemostaticefficacy was assessed for each case intra- and post-operatively by the surgeons and hematologists independently, utilizing objective 4-point ordinal efficacy scales. This assessment was additionally adjudicated by the IDMC. Safety and immunogenicity were monitored throughout the study.
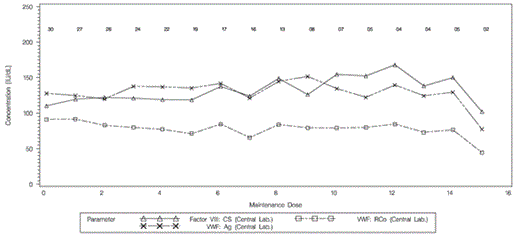
Twenty-eight individual patients, who underwent 30 surgeries, were enrolled from 14 sites in 8 countries in Europe, Asia, and the USA. Of the 30 procedures (2 patients had more than 1 procedure), 7 had VWD Type 1 unresponsive to DDAVP (23.3%); 2 had VWD Type 2 (6.7%, one with Type 2A and one with Type 2B), and 21 had severe VWD Type 3 (70.0%). Age at baseline ranged from 12 to 74 years (median 36 years), with 3 adolescents (younger than 18 years of age) included in the study. Most surgeries were either orthopedic (10 surgeries) or dental (7 surgeries). Five surgeries were obstetric/gynecological; 4 were gastrointestinal; 3 of the ear, nose or throat; and one was ophthalmologic. In total 21 major surgeries and 9 minor surgeries were performed. Of the 21 severe VWD Type 3 patients, 17 underwent major surgery and 4 underwent minor surgery. The mean loading dose of VWF/FVIII concentrate (Wilate) per infusion (measured in VWF:RCo units) was 55.5 IU/kg for major surgeries and 37.5 IU/kg for minor surgeries. The mean maintenance dose was 30 IU/kg for major and 20.6 IU/kg for minor surgeries. Mean FVIII:C and VWF:RCo peak plasma activity levels of each patient during the maintenance infusions (infusion 1 post surgery to infusion 7), ranged from 119 IU/dL – 138 IU/dL and 66 IU/dL - 92 IU/dL, respectively, and were stable over time with no accumulation of FVIII:C (Figure 1). In the 30 procedures patients received a mean total dose (VWF:RCo) of Wilate of 434.2 ± 271.2 IU/kg (29,086 ± 17,932 IU).
The overall treatment with Wilate was successful in 29 out of 30 surgeries (rate 0.967; 98.75 CI: 0.784, 1.000). Nine of 9 minor surgeries (success rate 1.000; 98.75% CI: 0.569, 1.000), and 20 of 21 major surgeries (success rate 0.952; 98.75% CI: 0.704, 1.000), were successful, as were all 21 surgeries in patients with Type 3 VWD (success rate 1.000; 98.75% CI: 0.785, 1.000).
None of the patients experienced excessive intra- or post-operatively bleeding that was uncontrolled or required an alternate VWF/FVIII concentrate. No incidences of thrombotic events were reported despite 1/3 of the surgeries being orthopedic and high risk for such in non-coagulopathic populations.
No FVIII or VWF neutralizing inhibitors or study drug related serious adverse events (SAEs) were observed.
Mean Course of Peak Values by Maintenance Dose for VWF:RCo, VWF:Ag and FVIII:C Concentrations (IU/dL) (Population, N=30)
Mean Course of Peak Values by Maintenance Dose for VWF:RCo, VWF:Ag and FVIII:C Concentrations (IU/dL) (Population, N=30)
Numbers above the graph indicate the number of observations at each dosing point
Conclusion: Results of this study showed that more than 96% of the study’s surgeries treated prophylactically with Wilate were rated as having excellent or good hemostatic efficacy, as adjudicated by the IDMC. The study was terminated early because of clear and robust demonstration of efficacy, as pre-specified in the protocol. There was no accumulation of FVIII:C activity after repeat dosing. No cases of VWF or FVIII:C inhibitors, thrombotic events, or related SAEs were observed.
Srivastava:Octapharma: Consultancy, Other. Off Label Use: Wilate is a Human VWF/FVIII product licensed in the US for treatment of acute bleeding in VWD patients but not licensed for prophylactic treatment in surgical procedures. Werner:Octapharma: Employment. Schwartz:Octapharma: Employment. Knaub:Octapharma: Employment.
Author notes
Asterisk with author names denotes non-ASH members.
This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal