Abstract
In acute myeloid leukemia (AML), residual leukemic (stem) cells that escape initial chemotherapy are considered a major source of relapse. Clinical trials have used IL-2 for AML patients with the aim to reduce relapse rates by eliminating residual leukemic cells through activation of NK and T cells. However, monotherapy with IL-2 has led to disappointing results. Nevertheless, recent clinical trials have shown that the co-administration of IL-2 and histamine dihydrochloride (HDC) provides maintenance of remission in AML. Histamine suppresses the formation of reactive oxygen species (ROS) thereby protecting NK and T cells from ROS-induced dysfunction and apoptosis. In addition, IL-2 is considered to maintain the anti-leukemic activity of NK cells. However, the direct effect of this treatment on NK cell numbers and anti-AML activity has not been studied in detail so far.
In this study, we analyzed the immunophenotype and function of NK cells in a cohort of 7 AML patients (FAB M2, n=1; M4, n=2; M4-eo with inv 16, n=2; M5, n=2) treated with HDC plus IL-2. All patients had received induction chemotherapy with daunorubicin (45 mg/m² i.v. days 1-3), cytosine arabinoside, ARA-C (2 x 100 mg/m² i.v., days 1-7) and etoposide (100 mg/m² i.v., days 1-5) as well as at least 3 cycles of consolidation chemotherapy with high dose or intermediate dose ARA-C (Sperr et al, Clin Cancer Res 2004;10:3965-3971 and Krauth et al, J Immunol 2006;176:1759-1768). After having achieved a complete hematologic remission, patients were treated with HDC (0.5 mg) plus recombinant IL-2 (0.9 x 106 IU) twice daily s.c. for 21 days per cycle. Blood was drawn before and during treatment with HDC plus IL-2.
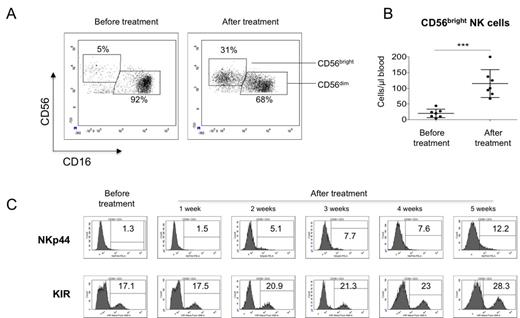
We found that after one week of treatment with HDC plus IL-2, NK cell numbers increased in peripheral blood (from 101.8 ± 28.25 cells/µl before therapy to 208.2 ± 38.27 cells/µl after therapy, p<0.05). In the NK cell fraction, we observed an astonishing increment of CD56bright NK cells in all treated patients (from 7.2±0.97% or 17.6±5.8 cells/µl before therapy to 38.8±4.4% or 104±19.4 cells/µl after therapy, p<0.05; see Fig.1A/B). The cytotoxic activity of the CD56bright cells, as determined by NK cell degranulation and target cell lysis using the cell line K562, showed a significant increase in comparison to cells obtained before treatment (p<0.05). This was associated with an increased expression of KIR as well as the activation markers NKp44 (see Fig.1C), NKp46, and CD25 on NK cells. Furthermore, we observed a significant increase in expression of CD56 on NK cells after treatment with HDC plus IL-2 in our AML patients (2.5 ± 0.55 fold increase in the mean fluorescence intensity of CD56, p=0.02), whereas CD16 expression did not change significantly. In addition, treatment with HDC+IL-2 also induced an increased proportion of circulating CD4+CD25highCD127low/neg regulatory T cells (Treg). Finally, in vitro stimulation of NK cells with histamine plus IL-2 mimicked the effects observed in vivo. Interestingly, the in vitro treatment was also associated with an increased expression of CD56 without altered expression of CD16, suggesting that this effect could be a specific and reliable indicator of in vivo responses of NK cells to HDC plus IL-2 therapy.
In conclusion, treatment with HDC plus IL-2 causes a striking increase in CD56bright NK cells. These specifically expanded NK cells exhibit an activated phenotype with enhanced potential to kill leukemic cells. We propose that the maintenance of remission in AML patients treated with HDC plus IL-2 might, at least in part, be the result of an improved anti-leukemic NK cell function.
Effect of HDC plus IL2 on NK cells of AML patients. A) Representative dot plots of the CD56bright and CD56dim NK cell subpopulations from an AML patient treated with histamine+IL2 before and after treatment. B) Absolute cell numbers of CD56bright NK cells of 7 AML patients before and after treatment, *** p<0.01. C) Follow-up of NKp44 and KIR expression after HDC plus IL-2 therapy. Shown are histograms for NKp44 and KIR expression on total CD56+ CD3- NK cells of one patient representative for the majority of patients tested.
Effect of HDC plus IL2 on NK cells of AML patients. A) Representative dot plots of the CD56bright and CD56dim NK cell subpopulations from an AML patient treated with histamine+IL2 before and after treatment. B) Absolute cell numbers of CD56bright NK cells of 7 AML patients before and after treatment, *** p<0.01. C) Follow-up of NKp44 and KIR expression after HDC plus IL-2 therapy. Shown are histograms for NKp44 and KIR expression on total CD56+ CD3- NK cells of one patient representative for the majority of patients tested.
Sperr:MEDA Pharma GmbH & Co. KG: Speakers Bureau. Valent:MEDA Pharma GmbH & Co. KG: Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal