Abstract
INTRODUCTION: Erdheim-Chester Disease (ECD) is a rare hematologic disease manifesting as granulomatous or fibrotic infiltration of long bones and non-skeletal tissues by non-Langerhans foamy histiocytic (CD68+, CD1a-, S100-) cells. The clinical presentation can be diverse and any organ can be affected. It is estimated that less than 500 cases have been reported in the literature.
METHODS: We retrospectively reviewed the medical records of patients evaluated at Mayo Clinic from January 2001 to May 2014. A tissue biopsy review by pathologists at Mayo Clinic was necessary for inclusion in the study. In all cases, the diagnosis of ECD was confirmed using clinical criteria in conjunction with histopathologic findings. Clinical data such as patient characteristics, disease presentation, management strategies, treatment responses, and survival time were collected.
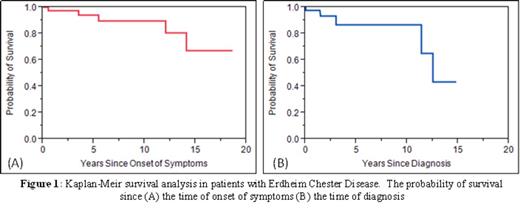
RESULTS: Forty-five patients with confirmed ECD were included in our study. The median age at diagnosis was 53 years (range, 32-78), while the median age of onset of symptoms prior to diagnosis was 24 months (range, 1-180). There was a male predilection with a male to female ratio of 2.4. The duration of follow-up ranged from 1-168 months (median, 20). At the time of last follow-up, 38 patients were alive and 5 were dead, while the vital statuses of 2 patients were unknown. The median and mean survival times from the time of diagnosis were 150 and 130 months respectively. The median survival time from the time of symptom onset was not reached and mean time was 154 months (Figures 1A and 1B).
Bone pain was the most common presenting symptom (27%). Other common presentations included diabetes insipidus (24%), abdominal pain (22%), B symptoms (13%), visual changes (13%), renal failure/obstruction (11%), pleural effusion (9%), other neurologic symptoms (9%), ataxia (7%), pericardial effusion (7%), edema (7%), dyspnea (7%), and hypertension (7%).
The organs/sites involved were bone (80%), central nervous system (59%), kidneys (56%), lungs (38%), retroperitoneum (33%), heart (22%), sinuses (22%), bone marrow (14%), adrenal glands (13%), liver (11%), spleen (11%), and skin (16%). Only 18 (40%) patients had the diagnosis established by the first biopsy. The rest required multiple biopsies before the diagnosis was confirmed (median, 2; range, 1-5). Testing for BRAF V600Ewas performed in 12 patients and 8 (67%) tested positive for the mutation.
Eight patients have not required ECD-specific treatment so far. Of the 37 patients who required treatment, the median number of treatments received was 2 (range, 1-7). The systemic treatments received and their response rates are summarized in Table 1. Four patients received radiotherapy but none responded. At the time of the last follow up, 5 patients with BRAFV600E were receiving treatment with vemurafenib or dabrafenib. However, follow-up is insufficient for proper assessment of response.
CONCLUSIONS: ECD is an extremely rare diagnosis as exemplified in our retrospective analysis. The diagnosis is often challenging due to protean and diverse clinical presentation as well as frequent inconclusive initial biopsies. There is usually a long-latency period of smoldering symptoms leading to definitive diagnosis. A high level of suspicion is essential in order to make the diagnosis and BRAFmutation analysis should be considered an integral part of work up even if biopsy is non-diagnostic. Traditional oncologic systemic therapies and even novel rheumatologic anti-inflammatory agents generally have limited objective activity. BRAF inhibitors offer a novel treatment option and results of prospective studies are eagerly awaited.
Summary of Response to Various Treatment Modalities in Patients with ECD
| Therapeutic Agent . | Number of Patients Treated . | Response (%) . | |||
|---|---|---|---|---|---|
| Complete Response . | Partial Response . | Stable Disease . | No response . | ||
| Corticosteroids | 11 | 0 | 0 | 9 | 91 |
| Methotrexate | 9 | 0 | 0 | 11 | 89 |
| Cyclophosphamide | 4 | 0 | 0 | 25 | 75 |
| 2-CDA | 12 | 8 | 17 | 25 | 50 |
| Interferon-α | 8 | 0 | 29 | 14 | 57 |
| Vinorelbine | 4 | 0 | 50 | 0 | 50 |
| Tumor necrosis-α factor Inhibitors | 7 | 0 | 0 | 14 | 86 |
| Interleukin-1 Receptor Antagonists | 6 | 17 | 17 | 0 | 67 |
| Tamoxifen | 2 | 0 | 50 | 0 | 50 |
| Radiotherapy | 4 | 0 | 0 | 0 | 100 |
| Therapeutic Agent . | Number of Patients Treated . | Response (%) . | |||
|---|---|---|---|---|---|
| Complete Response . | Partial Response . | Stable Disease . | No response . | ||
| Corticosteroids | 11 | 0 | 0 | 9 | 91 |
| Methotrexate | 9 | 0 | 0 | 11 | 89 |
| Cyclophosphamide | 4 | 0 | 0 | 25 | 75 |
| 2-CDA | 12 | 8 | 17 | 25 | 50 |
| Interferon-α | 8 | 0 | 29 | 14 | 57 |
| Vinorelbine | 4 | 0 | 50 | 0 | 50 |
| Tumor necrosis-α factor Inhibitors | 7 | 0 | 0 | 14 | 86 |
| Interleukin-1 Receptor Antagonists | 6 | 17 | 17 | 0 | 67 |
| Tamoxifen | 2 | 0 | 50 | 0 | 50 |
| Radiotherapy | 4 | 0 | 0 | 0 | 100 |
Off Label Use: We report among all treatments utilized at our institution for ECD including the use of steroids, immunosupressants, TNF blockers, interleukin-1 receptor antagonists, and BRAF inhibitors in patients with Erdheim Chester Disease. These treatments, though supported by other reports in the literature, have not been explicitly approved by the FDA for ECD..
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal