Abstract
Intro:
Dyserythropoiesis is a growing class of inherited diseases of various severities in which genes encoding for proteins involved in cellular trafficking, chromatin dynamic or transacting factors restricted to red cells maturation, are mutated. Belonging to this later groups, several mutation in GATA 1, an X linked transacting factor, were associated with dyserytropoietic anemia frequently associated with a thrombopenia.
Case report:
Our case is the second living child of a mother from Greek origin and a father from French origin. This couple had a first girl without any evidenced abnormality. During the second pregnancy, the male fetus had a severe anemia (Hb: 3.8 g/dL), regenerative (erythroblasts: 176 G/L). He received intrauterine transfusions but the worsening of anemia led to interrupt the pregnancy at 33 weeks. A third pregnancy was terminated because of a Down’s syndrome. A fourth pregnancy revealed the recurrence of a severe anemia at 27 weeks in a male fetus (Hb: 3.2 g/dl). Two intrauterine transfusions allowed the birth at 33 weeks of an anemic boy (at birth Hb 10.6 g/dL, reticulocytes: 261 G/L; erythroblasts: 647G/L). A myelogram was made when he was 3 months old, and showed a severe dyserythropoiesis, with a hypoplastic red cells lineage and a blockade on late erythroblasts. The megakaryocyte lineage appeared to be normal. He received Packed RBC up to 3 month, then Hb level stabilized spontaneously, with at the latest control, when he was aged 7 months, an Hb level at 10.9g/dl, MCV: 88 fL; reticulocytes: 152 G/L; circulating erythroblasts: 7%. Platelets count is 152 G/L. Leucocytes: 13.5 G/L, with PNN: 3.0 G/L, lymphocytes: 7.3 G/L, eosinophils: 1.4 G/L.
Genetic analysis in the family and cultivated cells:
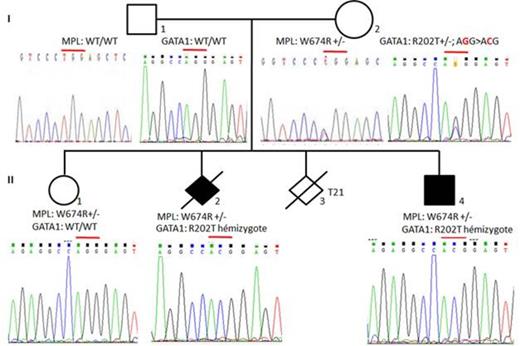
The available members of the family were extensively studied including globins locus (mutation and deletion), KLF 1, GATA 1, Bcl11a, CDN 1, sec 23B and MPL gene. The exome analysis of the living boy revealed no other genetic defect. In order to get evidence on the pathogenic consequence of the GATA-1 mutation, mRNA were extracted from BFUE obtained from the mother and were searched for the mutation (PCR digestion using Ade I/Dra III and Sanger sequencing) seeking for a "non random" X inactivation
Results:
Two locus were found to carry a mutation: GATA-1, p.Arg202Thr, NM_00249.3 (GATA-1):c.605G>C) and MPL: p.Trp474Arg, NM_005373.2(MPL_v001):c.1420T>C). The terminated fetus (male) and the living boy were found to be heterozygous for the MPL mutant and hemizygous for the GATA-1 mutant both mutations are inherited from the mother. and the sister is only heterozygous for the MPL mutant (fig 1). The father carries no mutation. The mRNA analysis of independent BFUE clones showed the presence of the wild type GATA-1 messenger into 26 from 28 colonies analysed ( 30 clones, 2 PCR fail).
Discussion and conclusion:
GATA -1 is a zinc finger transacting factor know to be necessary for the late erythroblasts maturation through the binding onto specific DNA targets with the Cter ZF (aa 258 to 293) and the interaction with FOG with Nter ZF (aa 202 to 248). Mutations affecting the Cter zinc finger would prevent the DNA binding and thus should result in a lethal phenotype as it was described in GATA-1 KO mice model. Several GATA-1 mutations involving the Nter ZF are described (M205V, G208R, G208S, D218G) and are involved in dyserythropoiesis usually with mild anemia. Here we describe a new mutation affecting a highly conserved aa belonging to the Nter ZF. In hemizygous patients, it produces a phenotype very close to that of cases previously described, but more restricted to the erythroid lineage as the only consequence on the thrombopoiesis is, up to now, only a small decrease in platelets count. This case may indicate that the functions of GATA-1 / FOG may differ between fetal and adult erythropoiesis as anemia seems to be worse in the fetal period. However, we evidenced a strong skewed X-chromosome inactivation toward wild type allele in the mother's erythroblasts which is a strong argument for a marked impairment of the interaction and/or the function of the GATA-1Arg202Thr /FOG complex.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal