Abstract
Imatinib (IM) failure in chronic phase CML patients is a peculiar situation where the quality of life (QoL) of patients has rarely been appreciated and assessed. A majority of patients is rescued by second generation tyrosine kinase inhibitors (TKI2) licensed in this setting. In the retrospective and prospective national observational POSTIM study we have already reported the value of the Hammersmith score in this population of patients. In the same population, we have also have prospectively analysed the QoL and the compliance of these IM-resistant or intolerant patients, on TKI2 (i. e. Nilotinib and Dasatinib) as a secondary objective, using a series of scores currently used to assess such characteristics (Morisky score, Functional Assessment of Cancer Therapy (FACT) subdivided in Physical Well Being (PWB), Social/Family Well Being (SWB), Emotional Well Being (EWB), Functional Well Being (FWB), Social/Family well being (SAC), (FACT+SAC=FACT-total), and FACT-Leu) calculated at 6 months and 12 months following first TKI2 initiation. These scores have been correlated to general characteristics of the patients, cytogenetic and molecular responses to TKI2 at one year of TKI2, and to survival.
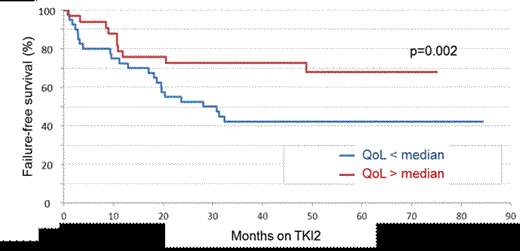
Among the 174 patients enrolled in the POSTIM study, 76 patients have been enrolled in this QoL study in 16 university and non-university academic institutions between 2009 and 2012, and their data collected after informed consent. There were 36 males (47%) and 40 females with a median age at enrolment of 62 (25-86) years and a median duration of CML of 6.5 (3-18) years. Sokal score was low for 37%, intermediate for 28% and high for 35 % (6 pts unknown). IM was stopped because of IM-resistance in 41% (n=29) of the patients, IM-intolerance in 40% (n=28), IM-intolerance + resistance in 19% (n=13) of the patients (6 patients unknown). None of the patients had been allotransplanted previously. Eleven % (n=6) of the patients had a high Hammersmith score (HS), 20% (n=11) an intermediate HS, and 69% (n=37) a low HS of evaluable patients (22 patients non evaluable). Sixty-one percent of the patients (n=46) had Dasatinib and 39% (n=30) had nilotinib as a first TKI2. The first TKI2 has been stopped for first TKI2 resistance (7 patients), intolerance (13 patients) and for resistance and intolerance (3 patients). Eleven patients went from Dasatinib to Nilotinib and 18 from Nilotinib to Dasatinib. The median follow-up after initiation of the first TKI2 is 4.5 (2.5-7) years. There was no difference in PWB, SWB, EWB, FWB, and FACT between Dasatinib and Nilotinib groups (p=0.56, 0.36, 0.53, 0.70, 0.66 respectively). Only one patient died, therefore the overall survival analysis was not relevant. Thirty-four patients (45%) failed the TKI2 treatment. We went on analysing the failure free survival [(FFS), failure defined as no hematologic or cytogenetic response, CHR, CCyR, PCyR MMR or MR4.5 loss, death, progression to AP/BC, definitive TKI2 cessation for resistance or intolerance, allogeneic stem cell transplantation]. Cox model analysis demonstrated that FFS was significantly longer in patients with a high FACT score since TKI2 initiation (median not reached for high FACT versus 28 months for low FACT score, p=0.02, see figure 1) and since CML diagnosis (median 161 months for high FACT versus 66 months for low FACT score, p=0.002; median 162 months for high FACT-total versus 87.5 months for low FACT-total score, p=0.012). In addition, the FFS since diagnosis was significantly better for patients with a high PWB score (median 161 months for high PWB versus 66 months for low PWB score, p=0.032). No difference in cytogenetic and molecular responses to TKI2 observed at 1 year have been influenced by any of the scores individually, or grouped (= FACT, FACT-total), neither the Morisky score.
In conclusion, this prospective analysis performed on a large population of patients failing imatinib, on TKI2, demonstrated that maintaining a good QoL for these patients evaluated through the FACT scores, can effectively improve failure-free survival on these drugs, and this needs to be a matter of concern for improving the long-term follow-up and the compliance to treatments of such patients.
FFS since TKI2 initiation according to the FACT questionnaire result (Patients have been split into 2 groups according to the median value of the score).
FFS since TKI2 initiation according to the FACT questionnaire result (Patients have been split into 2 groups according to the median value of the score).
Nicolini:Novartis: Consultancy, Honoraria; BMS: Consultancy, Honoraria. Roy:Novartis: Honoraria; BMS: Honoraria. Guerci-Bresler:Novartis, BMS, Pfizer: Honoraria. Legros:Novartis, BMS: Honoraria. Gardembas:BMS: Honoraria. Le Maux:BMS: Employment. Guinard-Azadian:BMS: Employment. Mohamed:BMS: Employment. Etienne:Novartis, BMS, Pfizer, Ariad: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal