Abstract
Background: Rituximab has increased the CR rate, improved both PFS and OS and changed the pattern of relapse of DLBCL pts treated with R-CHOP (leading to a > 50% cure). In contrast to the pre rituximab era, the majority of failures occur early (80% of failures occur in the 1st 18 months). The observation made that few relapses (7-8%) occur after 24 months of pts enrolled in clinical trials needs validation in the community setting. We report here a large cohort of pts treated at our institution over a period of 7 years with rituximab-containing chemotherapy regimens.
Methods:We performed a retrospective cohort analysis to describe the survival experiences of adult patients with de novo DLBCL treated at our institution between 2007 and 2013. Patients were identified using Hematopathology and John Theurer Cancer Center outcomes databases. Patients who didn’t receive rituximab as part of their initial combination chemotherapy were excluded, as well as transformed DLBCL, primary CNS DLBCL, HIV-related DLBCL pts, those lacking follow up data.
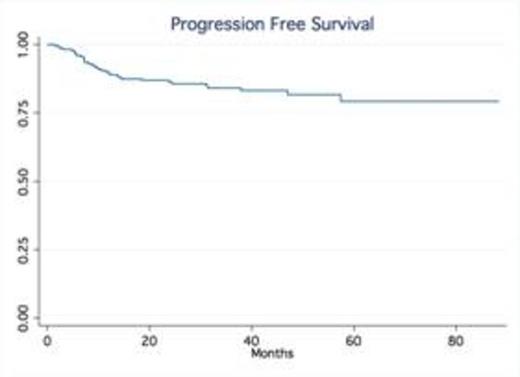
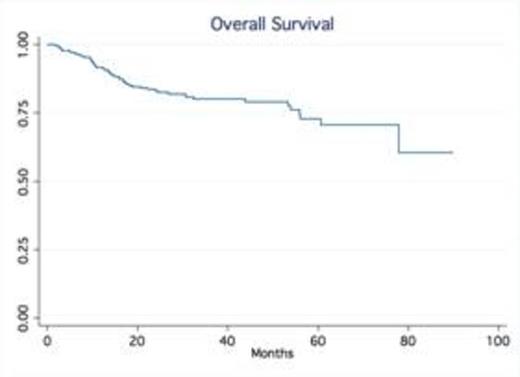
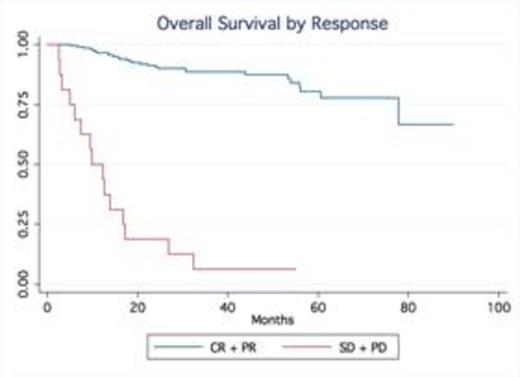
Results: A total of 245 patients with DLBCL treated at our institution were identified. Baseline characteristics were as follows: median age was 63 (20-92), 53% males, 75% stage III-IV and 24% stage I-II; IPI score was high in 25% of pts, high-int in 22%, low-int in 45% and low in 7%. Most patients received R-CHOP (66%), while 34% were treated with dose intense regimens based on high risk features such as high IPI, Ki-67 over 80% (R-HyperCVAD, DA-R-EPOCH, R-Magrath), only two patients had frontline planned stem cell transplant. 91% of the patients achieved a CR, 7% were primary refractory (progressed during therapy), 2% PR/SD ). With a median follow up time of 32.5 months, the median OS and PFS have not been reached (75% of patients are alive at 55.9 months). In contrast, the median OS of relapsed patients was only 14.4 months No gender-specific differences in survival were observed. No differences in PFS and OS were observed based on frontline chemotherapy regimen. Achieving CR significantly determines survival (p.<05, LR test). Relapses occurred within first 24 months in 54 out of total of 60 patients who relapsed after frontline therapy.
Conclusion: Our series confirm outside clinical trial setting that in contrast to the pre-rituximab era, late relapses are rarely observed in patients treated with rituximab–containing induction therapy. The majority of relapses occur within the first two years of the initial therapy. Our findings validate the data from the Mayo clinic experience (Maurer et al, JCO 2014). This observation not only provides reassurance to patients but also support the current imaging guidelines with no longer a need for routine imaging surveillance for relapse in DLBCL beyond 2 years after frontline therapy.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal