Abstract
Allogeneic HCT from a CCR5 Δ32/Δ32 unrelated donor by Hutter et al provides the only evidence to date of long term control of HIV infection, and a compelling argument in favor of homozygous CCR5 Δ32reconstitution as the key mechanism driving resistance to infection 6 years after stopping antiretroviral therapy (ART). Low prevalence of Δ32/Δ32 genotype (<1%) and stringent HLA criteria for conventional donors made the search for Òpatient number 2Ó unsuccessful for years. Here, we describe a case of allogeneic HCT using CCR5 Δ32/Δ32 cord blood (CB) cells in a patient with diffuse large B-cell lymphoma (DLBCL) and HIV infection.
A 36 year-old man with HIV-1 infection from 2009 was diagnosed with DLBCL stage IIA in 2012. The patient had no illnesses associated with AIDS, good disease control and adherence to ART, with <50 HIV-1 RNA copies/mL and 360 CD4 T-cells/μL at DLBCL onset. Prior to HCT, the patient received CHOP-R (x6), ESHAP-R (x3), an autologous transplant with BEAM conditioning, GEMOX (x3), and abdominal radiotherapy. At HCT the patient had a refractory lesion in the left psoas, no B symptoms and good clinical performance. Baseline HIV-1 latent reservoir tests showed DNA copies in bulk and resting CD4 T cells and in GALT, CD4 T cell-associated HIV-1 RNA, free HIV-1 RNA in cerebrospinal fluid, replication competent viral size of 1.2 copies/107 CD4 T cells, and SCA (single copy assay) of 303 copies/mL. Patient's HIV-1 was CCR5 tropic by both genotypic and phenotypic analyses.
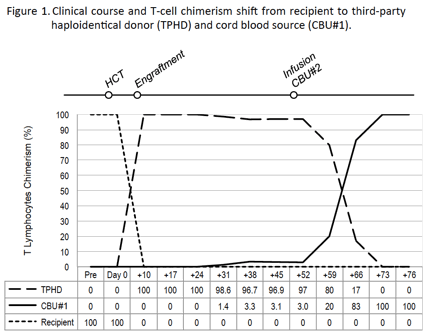
No HLA-matched sibling donors were available. Two CCR5 Δ32/Δ32 CB units (CBU) identified at the StemCyte inventory (Covina, CA) were HLA-compatible with the patient at the 4/6 level. A myeloablative CB alloHCT was performed using the single CCR5 Δ32/Δ32 CBU with higher cellularity (#1; Table 1) plus CD34+ cells from a haploidentical brother. The patient provided informed consent and IRB approved the alloHCT and research protocol. During HCT, ART combined abacavir, lamivudine and raltegravir. Neutrophil engraftment, 100% derived from the haploidentical donor, occurred on day +11. Post-thawing analysis of CBU#1 showed very poor clonogenic capacity, and donor switch to CB-origin failed, with persistently low CB-chimera <5% for up to 7 weeks (Figure 1). CBU#2 was infused on day +52, leading to a very rapid increase in CBU#1 chimerism, to 20% by day +59, 83% by day +66 and 100% by day +73. At this point, the patient's hematopoiesis, including CD4 T-lymphocytes, became CCR5 Δ32/Δ32. Ultrasensitive plasma viral load analysis decreased right after HCT, reaching minimal levels at the time of full CB-chimera. No HIV-1 DNA was detected using droplet digital PCR quantification or semiquantitative tests of amplification. In addition, although recipient CD4 T cells responded to proliferation and activation stimuli, they were resistant to infection by the patient's viral isolate as well as laboratory-adapted HIV-1 strains. Regretfully, our patient developed an aggressive DLBCL progression, and following very rapid clinical deterioration died from disease progression three months after HCT.
Long term monitoring and target organs and tissues analysis of viral reservoir will not be possible in this case. However, our data suggest that CCR5 Δ32/Δ32 CB HCT can successfully eliminate HIV-1 and render the recipient's T cells resistant to HIV-infection. Thus, they strongly support the use of CB as a platform for a broader application of this curative technology to other HIV-1 infected individuals.
Patient, Donor and Cell Product Characteristics
| . | Recipient . | Haploidentical Brother . | CBU #1 . | CBU #2 . | ||
|---|---|---|---|---|---|---|
| Year of birth or collection | 1975 | 1971 | 2008 | 2008 | ||
| CCR5 genotype | WT / WT | WT / Δ32 | Δ32 / Δ32 | Δ32 / Δ32 | ||
| HLA typing | ||||||
| - HLA A | 01:01 / 68:01 | 11 / 68 | 01:01 / 03:01 | 01:01 / 02:XX | ||
| - HLA B | 08:01 / 07:02 | 35 / 07 | 08:01 / 07:02 | 08:01 / 07:02 | ||
| - HLA DR | 03:01 / 13:01 | 01 / 13 | 03:01 / 14:01 | 03:01 / 14:01 | ||
| Cell products content | Pre-HCT Controls | Post Thawing # | Pre-HCT Controls | Post Thawing # | ||
| CD34 (x105/kg) | − | 40 ¤ | 0.7 | 0.52 | 0.73 | 0.22 |
| TNC (x107/kg) | − | − | 2.53 | 2.05 | 1.98 | 1.64 |
| CFU-GM (x104/kg) | − | − | 1.02 * | 0.11 | 0.12 * | Nil |
| Total CFU (x104/kg) | − | − | 2.13 * | 0.21 | 0.42 * | Nil |
| ¤ Purified by Miltenyi positive selection to include <1 x104/kg CD3+ cells; * Quality controls performed prior to HCT at the transplant center's laboratory in aliquot samples from segments attached to the units, as data were not available in the reports from the cord blood bank; # Subsequent quality controls in the final cell product after thawing the CBU for infusion. | ||||||
| . | Recipient . | Haploidentical Brother . | CBU #1 . | CBU #2 . | ||
|---|---|---|---|---|---|---|
| Year of birth or collection | 1975 | 1971 | 2008 | 2008 | ||
| CCR5 genotype | WT / WT | WT / Δ32 | Δ32 / Δ32 | Δ32 / Δ32 | ||
| HLA typing | ||||||
| - HLA A | 01:01 / 68:01 | 11 / 68 | 01:01 / 03:01 | 01:01 / 02:XX | ||
| - HLA B | 08:01 / 07:02 | 35 / 07 | 08:01 / 07:02 | 08:01 / 07:02 | ||
| - HLA DR | 03:01 / 13:01 | 01 / 13 | 03:01 / 14:01 | 03:01 / 14:01 | ||
| Cell products content | Pre-HCT Controls | Post Thawing # | Pre-HCT Controls | Post Thawing # | ||
| CD34 (x105/kg) | − | 40 ¤ | 0.7 | 0.52 | 0.73 | 0.22 |
| TNC (x107/kg) | − | − | 2.53 | 2.05 | 1.98 | 1.64 |
| CFU-GM (x104/kg) | − | − | 1.02 * | 0.11 | 0.12 * | Nil |
| Total CFU (x104/kg) | − | − | 2.13 * | 0.21 | 0.42 * | Nil |
| ¤ Purified by Miltenyi positive selection to include <1 x104/kg CD3+ cells; * Quality controls performed prior to HCT at the transplant center's laboratory in aliquot samples from segments attached to the units, as data were not available in the reports from the cord blood bank; # Subsequent quality controls in the final cell product after thawing the CBU for infusion. | ||||||
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal