Abstract
Background: The most common cause of treatment failure after reduced intensity conditioned allogeneic stem-cell transplantation (RIC alloSCT) is disease relapse. The curative potential of RIC transplants relies primarily on the immunologic graft-versus-tumor (GvT) effect. We hypothesized that graft T-cell doses correlate with relapse and survival outcomes, and that the graft T-cell content could be predicted according to donor characteristics.
Methods: We retrospectively studied 218 consecutive patients (pts) who underwent a first peripheral blood alloSCT after fludarabine (120 mg/m2) and busulfan (6.4 mg/kg) conditioning between 2006 and 2014, of whom 190 pts had available graft T-cell content data. CD3, CD4, CD8 and CD34 doses were analyzed. Cox regression was used to evaluate correlations between cell doses and time-to-relapse, relapse-free survival (RFS), overall survival (OS), and acute and chronic graft-versus-host disease (GvHD). Standard variables and the Disease Risk Index (DRI) were used for adjustment in multivariate models. A Classification and Regression Tree (CART) procedure was used to identify optimal cutoffs for continuous variables that correlated with outcome. Finally, we studied 23 alloHSCT donor blood samples to identify clinical and immunophenotypic characteristics that correlate with graft T cell content.
Results
The median follow-up was 29.6 mo. (range 0.4 – 81.5). The median age was 62 (range 21-76) and diseases included AML (79), MDS (42), NHL (31), CLL (8), ALL (10) and others (20). Pts were allografted from HLA-matched (86%) or mismatched (14%), sibling (43%) or unrelated (57%) donors. There was significant variability in T-cell doses: CD3 - mean 2.3x108/kg (range 0.2 – 8.1), CD8 - mean 0.5x108/kg (range 0.03 – 2.2), CD4 - mean 1.3x108/kg (range 0.1 – 5.4).
Relapse and survival: A high CD8 cell dose correlated with a decreased risk of relapse and improved RFS and OS. The univariate hazard ratios (HR) per 1 x 108/kg CD8 cells were: relapse 0.44 (95% CI [0.24 – 0.83]), p = 0.01; RFS 0.50 (95% CI [0.30 – 0.83]), p = 0.008; OS 0.63 (95% CI [0.37 – 1.08]), p = 0.09. After adjustment for DRI, GvHD prophylaxis and age, the CD8 cell dose remained significantly correlated with relapse, RFS and OS (Table 1). There were no significant correlations between the CD34, CD3 or CD4 doses and these outcomes.
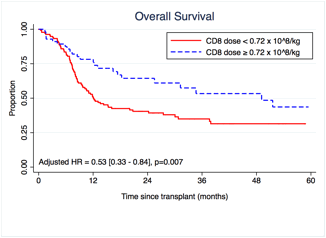
Using CART, we identified a cutoff of 0.72 x 108/kg CD8 cells that predicted OS. We found that pts who received a CD8hi graft had improved OS (adjusted HR = 0.53, 95% CI [0.33 – 0.84], p = 0.007; Fig. 1).
Engraftment: Donor T-cell engraftment correlated with higher CD8 but not CD4 cell doses. Donor T-cell chimerism levels were higher in pts who received a higher CD8 cell dose (day 30: r = 0.27, p = 0.002; day 60: r = 0.28, p = 0.005; day 100: r = 0.22, p = 0.01). Time to neutrophil or platelet engraftment was not affected by CD3, CD4 or CD8 doses although a high CD34 dose predicted faster neutrophil recovery (r = -0.19, p = 0.01).
GvHD: There were no significant correlations between T cell doses and GvHD. A trend was observed between acute GvHD and high CD4 but not CD8 cell doses (adjusted HR = 1.29, 95% CI [0.97 - 1.71], p = 0.08). A trend was also observed between moderate-severe chronic GvHD and high CD8 cell doses (adjusted HR = 1.93, 95% CI [0.90 - 4.10], p = 0.09).
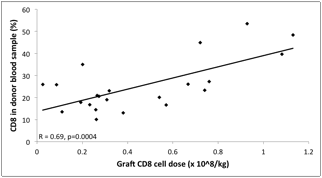
Donors: We investigated whether a high CD8 cell content could be predicted during donor screening. Not surprisingly, we found that donors whose grafts contained a high CD8 cell dose could be identified in a screening blood sample by having higher %CD8 cells (r = 0.69, p = 0.0004; Fig. 2), lower CD4/8 ratio (r = -0.55, p = 0.008) and higher %CD3 cells (r = 0.37, p = 0.09). There were no correlations between the graft content and other immunophenotypic parameters or clinical variables such as donor age, weight, gender or viral serologies.
Conclusion: A high CD8 cell dose correlates with improved RFS and OS after RIC alloSCT through a reduction in relapse without a significant increase in GvHD. Donor screening can potentially be optimized by selecting donors from whom a CD8-high graft can be collected. Targeting CD8 cell doses should be considered in prospective trials of RIC alloSCT.
Adjusted HRs of graft T-cell doses and outcomes
| . | Relapse . | RFS . | OS . | |||
|---|---|---|---|---|---|---|
| Variable | HR | p | HR | p | HR | p |
| CD8 dose | 0.43 | 0.011 | 0.49 | 0.006 | 0.57 | 0.046 |
| CD4 dose | 0.91 | 0.53 | 0.93 | 0.57 | 1.05 | 0.68 |
| CD3 dose | 0.85 | 0.15 | 0.87 | 0.12 | 0.94 | 0.49 |
| . | Relapse . | RFS . | OS . | |||
|---|---|---|---|---|---|---|
| Variable | HR | p | HR | p | HR | p |
| CD8 dose | 0.43 | 0.011 | 0.49 | 0.006 | 0.57 | 0.046 |
| CD4 dose | 0.91 | 0.53 | 0.93 | 0.57 | 1.05 | 0.68 |
| CD3 dose | 0.85 | 0.15 | 0.87 | 0.12 | 0.94 | 0.49 |
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal