Abstract
Introduction:
Haploidentical (HI) HSCT offers a curative option to patients (pts) who lack an HLA matched donor. In the 2-step approach, pts receive a relatively large, fixed T-cell dose (2 x 108/kg) followed 2 days later by cyclophosphamide (CY). CY eradicates only the alloreactive T-cells, thus inducing bidirectional tolerance. CD34-selected stem cells are then infused and are not exposed to CY. Unlike T-cell depleted approaches, the 2 step regimen allows for rapid immune recovery and lower infectious complications. Coupled with acceptable GVHD rates, this approach has been associated with low non-relapse mortality. Given the consistent T-cell dose utilized in all pts, we investigated the effects of the variable CD34 stem cells on clinical outcomes and immune recovery.
Methods:
We retrospectively analyzed data from 148 pts who underwent a 2-step approach to haploidentical peripheral blood HSCT at Thomas Jefferson University between February 2006 and February 2014. The myeloablative (MA) conditioning regimen consisted of 12 Gy of TBI administered over 4 days, while the reduced intensity conditioning (RIC) regimen consisted of fludarabine (30 mg/m2 D1-4) + cytarabine (2 gm/m2 D1-4)/or thiotepa (5 mg/kg D1-3) and a fraction of 2 Gy TBI (D6). Conditioning was followed by an infusion of 2 x 108 CD3+ cells/kg donor T cells (step 1). CY 60 mg/kg/d x 2 was given starting 2-3 days after the T cell infusion. A CD34 selected product was then infused (step 2). Tacrolimus and MMF were utilized for immune suppression. In a prior multivariate analysis in patients older than 60, we identified CD34 dose as affecting survival. Using recursive partitioning, a dose of 5.2 x106 was identified as the cutoff point demarcating differences in survival. This analysis compares differences in outcome in all patients who underwent the 2-step haploidentical HSCT regardless of age, using a cutoff CD34 dose of 5.2x106 to demarcate both groups.
Results:
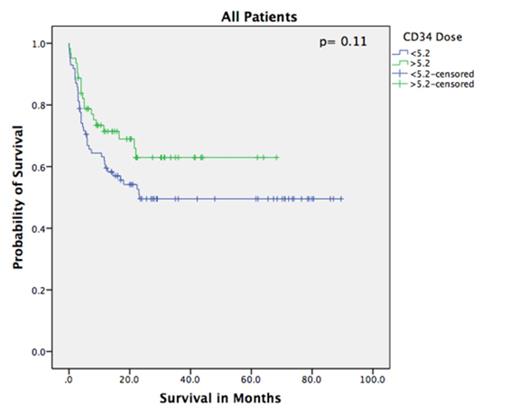
Eighty-five pts received a CD34 dose < 5.2 x 106(low dose- LD) and 61 received a dose > 5.2 x 108 (high dose- HD). Pts characteristics are shown in the table. Median follow up was 19 months. HD group had a faster platelet recovery (p=0.007) and more rapid CD3/4 and CD3/8 recovery by day 30 (p=0.001). The incidence of grades II-IV GVHD was not statistically different between both groups (p= 0.76). Probability of overall survival (OS) at 5 years was 50% and 62% in the LD and HD groups, respectively (log-rank= 0.14) with relapsed disease being the major cause of death in both groups. OS was significantly better in the HD in a subset of patients above the age of 60 (n=57, log-rank= 0.032). The 5-year cumulative incidence of relapse related mortality and non-relapse related mortality were not statistically significant between both groups; RRM: LD= 27%, HD= 20% (p=0.45); NRM: LD= 24%, HD=17% (p=0.32).
Conclusion:
Based on a platform of identical T cell dosing, the higher CD34 stem cell dose group had more rapid platelet engraftment, earlier immune recovery and better overall survival in a subset of patients above the age of 60. There were no differences in GVHD rates between both groups, which favors the use of a higher CD34 stem cell dose in this approach.
| . | Lower Dose (<5.2 x 106) . | Higher Dose (>5.2 x 106) . |
|---|---|---|
| Number | 85 | 61 |
| Age (range) | 58 (19-74) | 52 (19-78) |
| Sex (M/F) | 49/36 | 36/25 |
| Median CD3/4 day 30 (cells/ uL) | 34 | 71 |
| Median CD3/8 day 30 (cells/ uL) | 30 | 57 |
| Median CD34 cells [x 106/kg] (range) | 3.52 (1.4-5.18) | 7.31 (5.3-10.6) |
| Disease status at time of HSCT | ||
| Remission (%) | 38 (45) | 24 (39) |
| Active disease (%) | 47 (55) | 37 (61) |
| Disease | ||
| Myeloid Malignancy (%) | 58 (68) | 31 (51) |
| ALL (%) | 11 (13) | 11 (18) |
| NHL (%) | 11 (13) | 13 (21) |
| Others (%) | 5 (6) | 6 (10) |
| Conditioning | ||
| MA (%) | 52 (61) | 34 (56) |
| RIC (%) | 33 (39) | 27 (44) |
| Outcomes: | ||
| Median ANC recovery [days] | 12 | 11 |
| Median Platelet recovery [days] | 19 | 17 |
| aGVHD II-IV (%) | 33 (39) | 26 (43) |
| aGVHD III-IV (%) | 8 (9.4) | 4 (6.5) |
| cGVHD (%) | 14 (16) | 2 (3) |
| Relapse (%) | 25 (29.4) | 14 (23) |
| Deaths (%) | 41 (48) | 20 (33) |
| Relapse | 21 | 10 |
| Infection | 6 | 3 |
| Toxicity | 10 | 7 |
| GVHD | 4 | 0 |
| CMV Reactivation | 41 (48) | 25 (41) |
| . | Lower Dose (<5.2 x 106) . | Higher Dose (>5.2 x 106) . |
|---|---|---|
| Number | 85 | 61 |
| Age (range) | 58 (19-74) | 52 (19-78) |
| Sex (M/F) | 49/36 | 36/25 |
| Median CD3/4 day 30 (cells/ uL) | 34 | 71 |
| Median CD3/8 day 30 (cells/ uL) | 30 | 57 |
| Median CD34 cells [x 106/kg] (range) | 3.52 (1.4-5.18) | 7.31 (5.3-10.6) |
| Disease status at time of HSCT | ||
| Remission (%) | 38 (45) | 24 (39) |
| Active disease (%) | 47 (55) | 37 (61) |
| Disease | ||
| Myeloid Malignancy (%) | 58 (68) | 31 (51) |
| ALL (%) | 11 (13) | 11 (18) |
| NHL (%) | 11 (13) | 13 (21) |
| Others (%) | 5 (6) | 6 (10) |
| Conditioning | ||
| MA (%) | 52 (61) | 34 (56) |
| RIC (%) | 33 (39) | 27 (44) |
| Outcomes: | ||
| Median ANC recovery [days] | 12 | 11 |
| Median Platelet recovery [days] | 19 | 17 |
| aGVHD II-IV (%) | 33 (39) | 26 (43) |
| aGVHD III-IV (%) | 8 (9.4) | 4 (6.5) |
| cGVHD (%) | 14 (16) | 2 (3) |
| Relapse (%) | 25 (29.4) | 14 (23) |
| Deaths (%) | 41 (48) | 20 (33) |
| Relapse | 21 | 10 |
| Infection | 6 | 3 |
| Toxicity | 10 | 7 |
| GVHD | 4 | 0 |
| CMV Reactivation | 41 (48) | 25 (41) |
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal