Abstract
Introduction: Vitamin D3 (VitD3) plays a central role in calcium and bone homeostasis and has been shown to exert potent modulatory effects in both innate and adaptive immune responses. VitD3 deficiency is frequent in patients with hematopoietic malignancies undergoing allogeneic transplantation (alloSCT), and it has been implicated in the development of graft-versus-host disease (GVHD). However, the prognostic relevance of VitD3 in the context of alloSCT has not been fully delineated. This retrospective study investigates the prognostic impact of pre-transplant VitD3 levels on outcome parameters after alloSCT.
Patients and methods: Between 2001 and 2012, a total of 650 patients undergoing alloSCT at our institution (61% male, 39% female, median age 53 years) provided informed consent to participate in this observational study. 223 patients (34%) received transplants from related donors (RD), 281 patients (43%) from matched unrelated donors (MUD) and 139 (21%) from mismatched unrelated donors (MMUD). Diagnoses were acute myeloid leukemia in 202 patients (32%), lymphoid malignancies (lymphoma, chronic and acute lymphoid leukemia) in 228 patients (35%), multiple myeloma in 110 patients (17%) and other myeloid malignancies (including myelodysplastic syndrome, myelofibrosis and chronic myeloid leukemia) in 110 patients (17%). A total of 285 patients (44%) received statin treatment post alloSCT. Pre-transplant serum samples for VitD3 were collected between 0 and 2 months before alloSCT and cryopreserved at -80°C. VitD3 (25-hydroxyvitamin D3) concentrations were measured by an enzyme-linked immunosorbent assay (DiaSorin Deutschland GmbH, Dietzenbach, Germany). A total of 497 patients had serum samples prior to alloSCT available. In order to consider 153 missing VitD3 data sets multiple imputation (k=20) was applied. Cox regression analysis with cause specific hazard models was applied for overall survival (OS), non-relapse mortality (NRM) and time to relapse (TTR). As confounding prognostic factors we included tumour entity, statin treatment, VitD3, age, recipient's sex and donor as well as the interaction between VitD3 and donor, sex and statin treatment into the models. Survival times were measured from date of alloSCT.
Results: Estimated median follow-up time was 47.1 months. Median pre-transplant 25-hydroxyvitamin D3 level was 11.9 ng/mL (range 1-46.3 ng/mL). In multivariate analysis of the entire cohort, a significant interaction between recipient's sex and VitD3 level for OS (p=0.04) and TTR (p=0.03) could be observed. There was no interaction effect for NRM. Consequently, separate models for OS and TTR in male and female patients were performed.
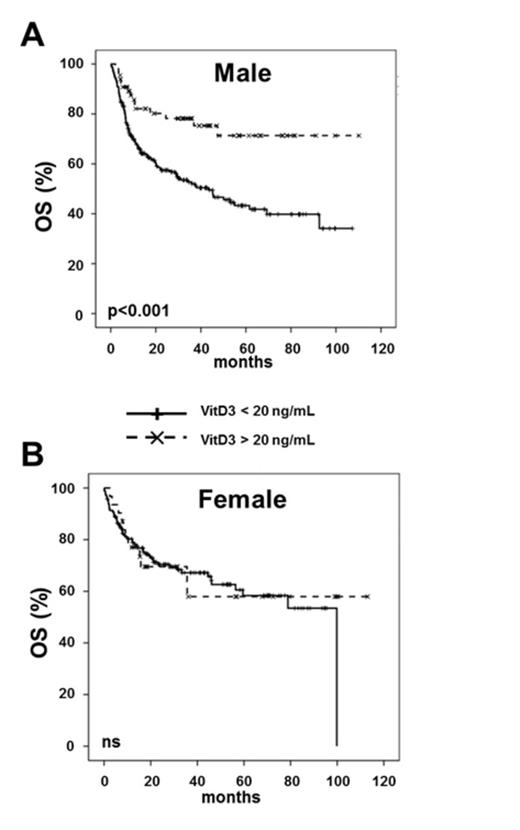
For female patients, no influence of VitD3 with regard to OS and TTR was observed. In contrast, Cox regression analysis of male patients revealed that lower VitD3 levels were significantly associated with inferior OS (HR=0.71 95%CI 0.55-0.91, p=0.008; hazard ratios were computed for an increase from the lower to the upper quartile of the distribution of VitD3). In male patients VitD3 was also significantly associated with TTR (HR=0.68 95%CI 0.50-0.93, p=0.015), i.e. longer time to relapse with higher VitD3 levels. Since guidelines recommend a VitD3 level of 20 ng/mL for the normal population, VitD3 levels were stratified at 20 ng/mL. In both sexes lower VitD3 levels did not affect NRM incidence. In male but not in female patients VitD3 levels below 20 ng/mL resulted in shorter TTR (p=0.001). Accordingly, VitD3 levels lower than 20 ng/mL were significantly associated with inferior OS in male (p<0.001) (Figure 1A) but not in female patients (Figure 1B).
Conclusion: This single center study suggests that male patients with lower pre-transplant VitD3 levels have higher relapse rates translating into a survival disadvantage across all diagnostic entities. Prospective studies investigating VitD3 in the alloSCT setting are highly warranted.
Radujkovic:DiaSorin Deutschland GmbH: 25-Hydroxyvitamin D3 ELISA kits were kindly provided by DiaSorin Deutschland GmbH, Dietzenbach, Germany. Other.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal