Abstract
Introduction: Double-unit cord blood transplantation (DCBT) is a viable therapy for adults with high-risk hematologic malignancies who lack an adult donor. However, lack of transfer of memory T cells in the graft is associated with an increased risk of viral infections. To study immune reconstitution, we recently described a novel method that combines 5' rapid amplification of complementary DNA ends (RACE) PCR and deep sequencing to quantify T cell receptor (TCR) diversity after allogeneic hematopoietic stem cell transplant (van Heijst, Nat Med 2013). In that study, we showed that recipients of DCBT recover TCR diversity comparable to healthy donors by 12 months. We now report results of a prospective analysis of CD4+ and CD8+ T cell repertoire recovery in DCBT recipients and correlation with clinical outcomes.
Methods: We prospectively collected samples from 33 DCBT recipients. The median age was 45 years (range 26-71), 18 (55%) were CMV seropositive, and the majority (n = 17, 52%) had non-European ancestry. Diagnoses included 20 (61%) acute leukemias and 13 (39%) lymphomas. Conditioning was myeloablative (n=1, 3%), reduced intensity (n=28, 85%), or non-myeloablative (n=4, 12%), and all patients received GVHD prophylaxis with cyclosporine-A and mycophenolate mofetil and no ATG. Patients received double-unit CB grafts (4-6/6 HLA-A,-B antigen, -DRB1 allele donor-recipient matched); this was supplemented with haploidentical CD34-selected PBSC in 18 patients. The 66 units had a median donor-recipient HLA-allele match of 6/8 (range 3-8). Infused total nucleated cell doses were 2.3 (1.7-3.3) and 1.9 (1.3-2.5) for the larger and smaller units, respectively. Samples were collected from the DCB grafts, recipient day+21 bone marrow, and peripheral blood at days +30, 60, 90, 120, 180 and 365 post-transplant. TCR-β sequences from each sample were amplified and sequenced using the Illumina/MiSeq sequencing platform after isolation of CD4+ and CD8+ T cells. TCR abundances were assessed at the level of clonotype and TCR diversity was calculated using inverse Simpson index.
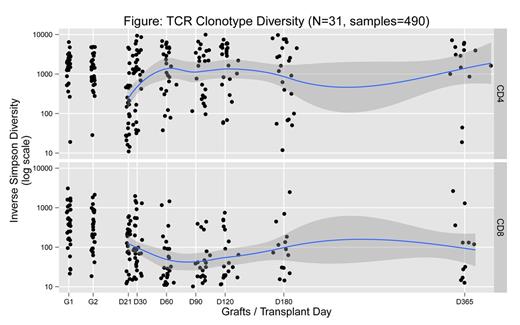
Results: Of the 33 patients, long-term samples were obtained in 25 patients, short-term samples (≤ day 100) in 6 patients who died early after DCBT, and no samples other than the graft for 2 patients. The remainder of the results focuses on 25 patients with complete samples. As previously shown, there is a 1-2-log increased diversity in CD4+ vs. CD8+ T cells (Figure). Furthermore, median CD4+ steady-state diversity is achieved early by day 60. In contrast, there is a higher rate of clonal dominance in CD8+ compared to CD4+ T cells (24/25 vs. 11/25, p=0.0001 by Fisher test). Several patterns of clonal dominance were observed, including two main patterns in CD8+ T cells. In 7/24 patients, clonal dominance is established by day 60 and persists throughout, whereas in 12/24 patients, clonal dominance fluctuates throughout follow-up. In CD4 +T cells, where less dominance is observed, a similar distribution is seen, though prolonged clonal dominance is rare. Interestingly, some of the dominant clones can be detected in the graft and are present in the day 21 marrow sample. Persistent clonal dominance in CD8+ T cells was seen 6/9 patients with CMV reactivation, whereas ongoing fluctuation was seen in 9/12 patients without CMV reactivation. In 2 patients with fluctuating clones who reactivated CMV, 1 had low level and the other a late viremia. In contrast, no link to a specific pattern was observed in patients with HHV6 viremia or acute GVHD. Finally, when we assessed similarity in clonal distribution between time points, there was more similarity in CD8+ than CD4+ T cells.
Conclusions: This novel deep TCR repertoire sequencing provides a quantitative picture of T cell recovery after DCBT and supports the following: 1) separate analysis of CD4+ and CD8+ T cell populations is critical given different patterns of recovery in T cell subsets; 2) there is significant turnover in CD4+ clones but with overall limited dominance, whereas there is less turnover in CD8+ clones; 3) although the grafts contain predominantly naïve T cells, the clonal evolution of CD8+ T cells strongly suggests generation of virus-specific T cells that control viral infection; and 4) CMV appears to be an important driver of CD8+ T cell clonal expansion after CBT. Ongoing analyses are correlating immune recovery with cord blood unit dominance as well as the biology of GVHD and relapse.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal