Abstract
Introduction: Hepatic veno-occlusive disease (VOD; also called sinusoidal obstruction syndrome) is a potentially fatal complication of hematopoietic stem cell transplantation (HSCT). Severe VOD (sVOD) is usually characterized by multi-organ failure (MOF) and >80% mortality. sVOD may develop in a substantial number of high risk patients (pts) and is often unpredictable. Defibrotide is an oligonucleotide that protects endothelial cells from transplant-induced damage and restores the thrombotic-fibrinolytic balance. Defibrotide is now approved in the EU for the treatment of severe hepatic VOD in HSCT. We report here the final results of a large, international compassionate use program (CUP), open from 1998-2009.
Methods: Defibrotide was provided on a compassionate basis or via single pt emergency-use requests (collectively, the CUP) to 312 sites in Europe, the US, Asia and the Middle East. There was no protocol; however, eligibility data, defibrotide use, safety and outcome data were requested using case record forms. In the US, pts were required to meet Baltimore criteria for VOD and have MOF. At all other sites, pts who met Seattle criteria for VOD, ultrasound, or histological criteria were eligible. The program included pts diagnosed with VOD as a complication of HSCT as well as chemo-/radiotherapy. Defibrotide was recommended to be administered IV in 4 divided doses daily, each dose infused over 2 hours. The initial dosing recommendations were 10 mg/kg/day with incremental dose increases of 10 mg/kg every 24 to 48 hours to a maximum daily dose of 60 mg/kg, depending on tolerability and response to treatment. Following results of a US Phase 2 study, the dose recommendation was 25 mg/kg/day. Duration of treatment was at the discretion of the investigator. Reported data included adverse events (AEs) and survival at Day+100 post HSCT or chemo-/radiotherapy.
Results: Enrollment data were provided for 1129 pts. Safety and outcome data (submitted voluntarily) were provided for 710 pts reported to have received ≥1 dose of defibrotide (433 male [61%]). Of these, 628 (89%) developed VOD following HSCT (499 [71%] allogeneic; 112 [16%] autologous; 17 [2%] other/missing) and 79 (11%) developed VOD following chemo-/radiotherapy alone; information on the index event that led to VOD was missing for 3 pts. The median age was 25 (range, 0.2-70) years; 303 (43%) were <18 years.
In the 710 pts, median time to onset of VOD from HSCT or start of chemo-/radiotherapy was 13 days. A total of 623 (88%) had bilirubin >2 mg/dL, 584 (82%) had weight gain >5%, 548 (77%) had hepatomegaly, 477 (67%) had ascites, 455 (64%) had right upper quadrant pain, and 292 (41%) had MOF. Per Bearman criteria or MOF, 429 (60%) had sVOD.
The median daily dose of defibrotide was 25 mg/kg given for a median of 15 days. The most common doses were 25 or 40 mg/kg/day (498 of 638 pts with dosing information, 78%).
AEs were reported in 378 pts (53%); most were serious (364/710, 51%) and fatal (350/710, 49%). Causes of death were generally reported as AEs, hence the most common new or worsening AEs were recorded as MOF (n=144 [20%], all fatal) and VOD (n=79 [11%], 78 fatal). Among the other AEs, sepsis (n=49 [7%], 48 fatal) was the most common. Incidence of serious AEs and fatalities was generally similar across dose subgroups. At least one hemorrhage event was reported by 85 (12%) pts (55 serious, including 37 fatal), most commonly gastrointestinal (n=33, 5%) or respiratory (n=24, 3%). Central nervous system (CNS) hemorrhage was reported in 10 pts (1%). Respiratory tract hemorrhage was highest in the 60/80 mg/kg/day dose group (9%) vs lower doses (<5%), but gastrointestinal and CNS hemorrhages were similar across dose groups (4%-6% and 0%-2%, respectively). Sixty three (9%) pts withdrew due to an AE, most due to hemorrhage (n=50, 7%), which was primarily gastrointestinal (n=22, 3%).
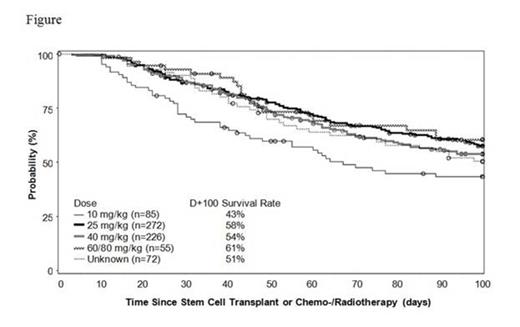
Across all doses in the 710 pts evaluated, the Kaplan-Meier (KM) estimate of survival at Day+100 was 54%. Figure shows KM estimated survival by dose group; in the 25 mg/kg/day subgroup (the approved EU dose) it was 58% at Day+100.
Conclusions: This large compassionate-use experience demonstrated that defibrotide was generally well tolerated and AEs were typical for this population. The overall KM estimated survival rate of 54% at Day+100 is consistent with prior studies. Results from subgroup analyses support the 25 mg/kg/day dosing as an optimal approach.
Support: Jazz Pharmaceuticals
Corbacioglu:Gentium: Consultancy, Honoraria. Off Label Use: Defibrotide is an investigational treatment for veno-occlusive disease in the United States.. Carreras:Gentium S.p.A.: Expert Testimony Other, Research Funding, Speakers Bureau. Mohty:Gentium S.p.A./Jazz Pharmaceuticals Inc.: Honoraria, Research Funding. Pagliuca:Gentium S.p.A.: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau. Ballabio:Jazz Pharmaceuticals Inc.: Employment, Equity Ownership. Hume:Jazz Pharmaceuticals Inc.: Employment. Bandiera:Gentium S.p.A.: Employment. Finetto:Gentium S.p.A.: Employment. Richardson:Gentium S.p.A.: Membership on an entity's Board of Directors or advisory committees; Jazz Pharmaceuticals Inc.: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.
This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal