Abstract
Introduction: Abnormalities involving chromosome 7 are common in myeloid stem cell neoplasms (MSN) including myelodysplastic syndromes (MDS) and acute myeloid leukemia (AML) and are associated with poor prognosis. Immunophenotyping by flow cytometry is an important tool to identify abnormal myeloid blasts and assist diagnosis of MSN. However, immunophenotypic features of MSN with monosomy 7 or deletion 7q have not been described. Here we retrospectively analyzed the flow cytometry data of MSN cases with monosomy 7 or deletion 7q and summarized a characteristic immunophenotype that could help distinguish chromosome 7 anomalies from other cytogenetic abnormalities.
Methods: Potential cases were identified by searching the UWMC pathology cytogenetic database from 2005 to 2013 for cases of MSN with monosomy 7 or deletion 7q detected by FISH or conventional G-banding cytogenetics. Only cases with concurrent flow cytometry analysis performed at UWMC Hematopathology Laboratory were included in this study. Flow cytometry was performed on a modified 4-laser, 10-color flow cytometer with the following antibodies used: CD4, CD5, CD7, CD13, CD14, CD15, CD16, CD19, CD33, CD34, CD38, CD45, CD56, CD64, CD71, CD117, CD123, and HLA-DR. Flow cytometry data were retrospectively analyzed to evaluate the antigen expression in myeloid blasts and maturing myelomonocytic cells.
Results: 126 cases of MSN harboring monosomy 7 and 95 cases harboring deletion 7q, either as a single anomaly or in combination with other cytogenetic abnormalities, were identified that had concurrent flow cytometry data. 112 cases with monosomy 7 and 73 cases with deletion 7q had adequate myelomonocytic cells for evaluation and were included in the study.
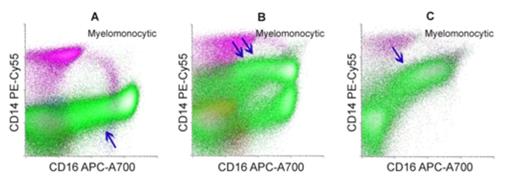
Increased CD14 on maturing myeloid cells was identified in 86 cases (77%) with monosomy 7. In addition to increased CD14 in the 86 cases, other immunophenotypic abnormalities were also identified, including presence of abnormal myeloid blasts (range 0-54%, median 7.3% of white cells); increased proportion of primitive blasts (CD34+/CD38-); aberrant expression of CD5 (22%), CD7 (72%), CD13 (48%), CD15 (19%), CD33 (50%), CD34 (37%), CD38 (57%), CD56 (41%), CD117 (54%), HLA-DR (59%) on the myeloid blasts; aberrant CD56 (43%) and CD64 (54%) expression on maturing myeloid cells; and aberrant CD56 expression on monocytes (41%). By contrast, only 6 cases with deletion 7q (8%) showed increased CD14 on maturing myeloid cells. Flow cytometry data from ten cases of regenerating marrow (day-28 marrow post therapy or post transplant) were also evaluated, and none of these cases showed increased CD14 on maturing myeloid cells. As previously reported, 10 of 15 cases receiving G-CSF therapy showed increased CD14 on myeloid cells, but without other immunophenotypic aberrancies.
Conclusion: Our data demonstrate MSN cases with monosomy 7 were associated with a characteristic immunophenotypic profile, including increased CD14 on maturing myeloid components and aberrant expression of multiple antigens on the myeloid blasts. The aberrant immunophenotypic pattern may help identify patients with monosomy 7 at diagnosis even in the absence of abnormal myeloid blasts and may potentially help to monitor response following treatment.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal