Abstract
Acute myeloid leukemia with normal cytogenetics (CN-AML) is biologically and clinically heterogeneous. Better prognostication and therapeutic strategies are required to improve outcomes. Evasion of host immunity is an important hallmark of cancer. Conversely, robust immune responses have been associated with improved outcomes in solid organ and hematological malignancies, for example, Hodgkin lymphoma. Moreover, targeting immune responses specifically at leukemic cells utilizing bi-specific T cell-engaging antibodies and chimeric antigen receptor T cell immunotherapy demonstrates efficacy primarily in lymphoid malignancies. We therefore sought to explore the effect of immune response on outcomes in myeloid cancers by aiming to determine whether T cell numbers in CN-AML at diagnosis would predict survival.
Using diagnostic trephine sections from patients with CN-AML at our institution between 2006 and 2013, we performed immunohistochemistry for CD3, CD8 and Granzyme B (GB) expression. CD4 was not assessable due to high background staining. For each trephine section, three representative fields at 200x magnification were analyzed with a mean of 4245 cells per field. Positive cells, enumerated using Fiji© image analysis software (v1.48o), were expressed as a percentage of total cells. The primary outcome was overall survival (OS). Cox regression was used for univariate and multivariate analyses. Survival was estimated by Kaplan-Meier analyses and categories compared using the log-rank test.
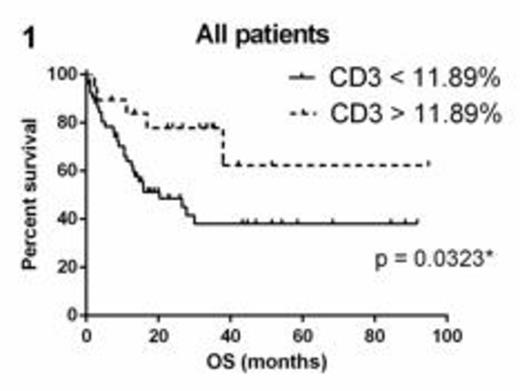
Seventy-five patients (52% male, median age 61 years) were analyzed with a median follow-up of 15.9 months. Fifty-six (75%) patients were treated with curative intent with 21 (28%) proceeding to allogeneic transplant either in first complete remission (CR1) (11 patients) or CR2 (10 patients). Of the 33 (44%) patients who died, 18 never achieved CR and 15 relapsed including 3 patients who were allografted in CR1 and 3 patients allografted in CR2. OS was superior in patients with CD3% above the 75th centile (>11.89%) compared with those below (p = 0.0323) (Figure 1). Factors significantly associated with better OS on univariate analyses were younger age, allograft, absence of preceding myelodysplastic syndrome, absence of primary refractory disease, and FLT3-ITD negativity. CD3 (p=0.096), CD8, GB, gender, NPM1 positivity, relapse and blast percentage at diagnosis were not statistically significant. However, in a multivariate analysis of CD3 and the variables found to be significant in the univariate analyses, higher CD3 was found to be an independent predictor of OS (Hazard ratio 0.922 for death, 95% CI 0.851-0.998, p=0.045). Relapse-free survival was evaluable in 49 patients who achieved a CR and was not influenced by CD3, CD8 or GB expression.
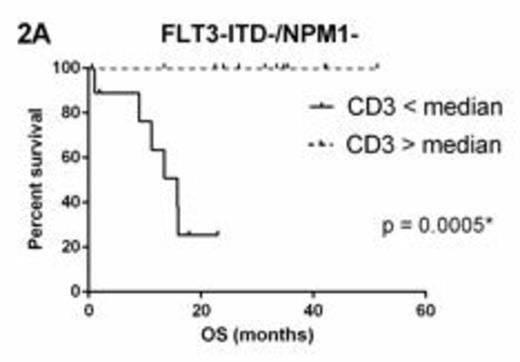
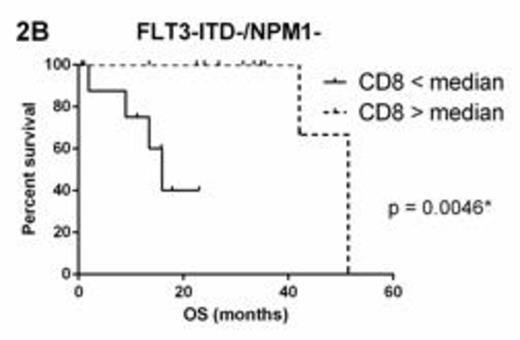
FLT3 and NPM1 status were available for 53 (71%) patients. Within molecular subgroups FLT3-ITD+ (n=20) and NPM1+/FLT3-ITD- (n=11), there was no survival difference between groups above and below the median for CD3, CD8 or GB. However, in FLT3-ITD-/NPM1- patients (n=22), CD3 > median (11.89%) (Figure 2A) and CD8 > median (10.66%) (Figure 2B) were associated with significantly superior OS whilst GB > median (1.17%) was not significantly associated (p=0.2330).
Our findings show that in CN-AML, especially in the FLT3-ITD-/NPM1- subgroup, higher CD3 T cell numbers are associated with improved survival. This suggests baseline immune status may have prognostic value. These results provide impetus for studies into immune therapies in AML and prospectively assessing baseline immune status as a potential prognostic marker in CN-AML.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal