Key Points
RTX treatment results in loss of human GC B cells.
Human Tfh and Tfr cells do not require GC B cells for their maintenance.
Abstract
The monoclonal anti-CD20 antibody rituximab (RTX) depletes B cells in the treatment of lymphoma and autoimmune disease, and contributes to alloantibody reduction in transplantation across immunologic barriers. The effects of RTX on T cells are less well described. T-follicular helper (Tfh) cells provide growth and differentiation signals to germinal center (GC) B cells to support antibody production, and suppressive T-follicular regulatory (Tfr) cells regulate this response. In mice, both Tfh and Tfr are absolutely dependent on B cells for their formation and on the GC for their maintenance. In this study, we demonstrate that RTX treatment results in a lack of GC B cells in human lymph nodes without affecting the Tfh or Tfr cell populations. These data demonstrate that human Tfh and Tfr do not require an ongoing GC response for their maintenance. The persistence of Tfh and Tfr following RTX treatment may permit rapid reconstitution of the pathological GC response once the B-cell pool begins to recover. Strategies for maintaining remission after RTX therapy will need to take this persistence of Tfh into account.
Introduction
In response to infection or immunization with a T-dependent antigen, germinal centers (GC) form within the B-cell follicles of secondary lymphoid tissues.1 GC are clusters of rapidly dividing B cells that are undergoing rounds of somatic hypermutation of their antigen receptor genes. This process of somatic hypermutation is random; therefore, in order to improve the affinity of cells that exit the GC as differentiated cells, selection needs to occur. B cells compete with each other for T-cell help within the GC; B cells with high-affinity for antigen can outcompete lower affinity B cells for T-cell help. Those B cells that receive help differentiate into antibody secreting plasma cells and memory B cells.2-4 T-cell help within the GC is provided by a subset of CD4+ T cells, or T-follicular helper (Tfh) cells. Tfh are a specialized subset of CD4+ helper T cells that migrate into GC and provide help and survival signals to GC B cells, promoting their differentiation into long-lived plasma or memory B cells.5,6 T-cell help is essential for the formation and maintenance of the GC and the response collapses in the absence of Tfh.7 The survival signals provided by Tfh to those GC B cells with the highest affinity B-cell receptor allow these B-cell clones to proliferate and differentiate to become the predominant antibody producing cells.8 Tfh are required for the response to foreign antigens, but in excess they can support autoreactive GC responses, leading to autoimmunity.9,10 In addition to Tfh, there is another subset of CD4+ T cells within the GC, T-follicular regulatory (Tfr) cells, that have been characterized in mice by our group and others.11-13 Tfr cells share phenotypic characteristics with Tfh but are derived from suppressive Foxp3+ regulatory T cells (Tregs). Tfr co-opt aspects of the Tfh differentiation pathway and upregulate B-cell lymphoma-6 (Bcl-6), the transcriptional repressor that is essential for the formation of Tfh.11,14-16 This allows Tfr to enter the GC and exert a suppressive function. Within the GC, Tfr cells control the size of the GC response and restrict the outgrowth of non–antigen-specific B-cell clones.11-13
The formation of Tfh and Tfr is dependent on interactions with B cells outside the B-cell follicle. Recent data suggests that the initial step in the formation of Tfh is upregulation of the achaete-scute homolog 2 (Ascl2).17 This transcription factor induces upregulation of the chemokine receptor CXCR5, the ligand of which, CXCL13, is expressed in the B-cell follicle, enabling pre-Tfh to migrate to the border of the B-cell follicle. Ascl2 has also been shown to suppress genes associated with other T-cell subsets, priming pre-Tfh differentiation down the follicular pathway.17 Pre-Tfh cells also express Bcl-6, which is both necessary and sufficient for Tfh differentiation.14-16 In contrast with the role for Ascl2 in Tfh cells, Tfr cells require NFAT2 for upregulation of CXCR5 and their subsequent migration.18 At the T-B border, Tfh precursors encounter antigen primed B cells and receive a second round of antigen presentation, enabling them to stabilize Bcl-6 expression, commit to becoming a Tfh cell, and migrate into the GC.19,20 In return, pre-Tfh provide signals to B cells to initiate immunoglobulin isotype class switching and form GCs.21 In mice, it is clear that the interactions between Tfh, Tfr, and GC B cells are reciprocal. Tfh and Tfr both require ongoing interactions with GC B cells in order to maintain their phenotype and function, and selective lack of GC B cells during an ongoing response leads to a reduction in Tfh numbers.22 Equally, GC B-cell numbers and differentiation depend on support from Tfh, with the GC response collapsing in the absence of Tfh.11-13,22
Translating the extensive knowledge of mouse Tfh and Tfr biology into humans has been difficult, in part because obtaining normal human secondary lymphoid tissue is not straightforward, but also because manipulation of the immune responses in humans is rarely possible. In humans, both Tfh and Tfr have been identified in tonsillar GCs, but description of their development and function has been limited.12,23-26 Here, we have taken advantage of the use of the B-cell depleting anti-CD20 monoclonal antibody rituximab27 (RTX) prior to renal transplantation to assess the effects of this treatment on lymph node GC, Tfh, and Tfr cells, and their circulating counterparts.
Because of the limited access to secondary lymphoid tissues from humans, several groups have examined circulating cells expressing the same surface markers as Tfh, in particular CXCR5, inducible costimulator (ICOS), and programmed cell death-1, coined circulating Tfh (cTfh)-like cells, that can act as a biomarker for Tfh differentiation in the secondary lymphoid tissues.25,28-30 cTfh numbers are elevated in patients with autoimmune diseases such as systemic lupus erythematosus31 and rheumatoid arthritis,32 and correlate with increased antibody levels and disease severity. cTfh-like cells appear to be a population of memory T cells that are generated during Tfh differentiation. Tfh-like cells do not express BCL-6 protein, but are able to quickly differentiate into cells capable of supporting antibody production.25,28-30 In order to compare cTfh-like cells with tissue resident cells, we have obtained paired blood and tissue samples from patients immediately prior to renal transplantation, who have been screened to exclude concurrent infection or malignancy. Some patients undergoing transplantation will have preformed antibodies directed against donor antigens, namely donor-specific antibodies.33 These can be antibodies against blood group antigens, or anti-human leukocyte antibodies formed from sensitizing events such as pregnancy. To reduce antibody titers and allow ABO or anti-human leukocyte antibody incompatible transplantation, patients can undergo “desensitization” regimens,33 often including the anti-CD20 monoclonal antibody RTX.27 RTX provides rapid and profound depletion of circulating B cells, although pre-B and mature plasma cells do not express CD20 and are thus preserved.34 In our center, RTX is given a month before transplantation along with tacrolimus and mycophenolate mofetil (MMF), then followed with five sessions of plasma exchange, with the aim of reducing donor-specific antibodies titers by targeting both the antibodies themselves and the cells that produce them.
We have taken advantage of the RTX treatment regimen to examine the importance of B cells in maintaining the Tfh and Tfr populations in human lymph nodes. We show that RTX in combination with tacrolimus and MMF is an efficient treatment for removing circulating naïve B cells, but not memory B cells, and surprisingly does not significantly alter the number of lymph node B cells. However, RTX in combination with tacrolimus and MMF leads to an absence of GC B cells in the lymph nodes of treated patients. Strikingly, despite the lack of GC B cells, Tfh and Tfr are still present, suggesting that, contrary to reports in the mouse, human Tfh and Tfr cells do not require the GC B-cell niche for their maintenance.
Patients and methods
Patients
This study received ethical approval from the Local Research Ethics Committee. Patients were recruited from the renal transplant live-donor program, and provided informed consent to participation in accordance with the Declaration of Helsinki. A total of 26 patients provided paired blood and tissue samples (9 women and 17 men). Mean age was 46 years (range, 18-69 years). All patients were either receiving or within 6 months of requiring renal replacement therapy. Patients taking immunosuppressive medication prior to transplant were excluded, except for those undergoing planned desensitization.
Patients undergoing desensitization therapy (n = 5) received 500 mg of the anti-CD20 monoclonal antibody (RTX) 1 month before transplantation, followed by tacrolimus (dose adjusted for each patient and aiming for blood levels of 5-15 ng/mL), and MMF 1 g twice daily. All patients underwent antibody removal with plasma exchange for 5 sessions prior to transplantation, which went ahead only if antibody levels were within locally acceptable limits.
At the time of transplant, 50 ml of blood was taken and lymphoid tissue was removed to allow access to iliac vessels as part of the routine operative procedure.
Sample preparation
Antibodies
Anti-human CD45RA clone HI100 (eBioscience), CD127 clone eBioRDR5 (eBioscience), CXCR3 clone IC6 (BD), FoxP3 clone PCH101 (eBioscience), CCR6 clone R6H1 (eBioscience), BCL-6 clone K112-91 (BD), CD57 clone NK-1 (BD), CD4 clone OKT4 (eBioscience), CXCR5 clone RF8B2 (BD) CD20 clone 2H7 (eBioscience), CD19 clone SJ25C1 (eBioscience), CD38 clone HIT2 (BD), IgD clone 1A6-2 (BD), CD27 clone O323 (eBioscience), and Streptavidin AF780 (eBioscience) were used.
Tfh, Tfr, and B-cell cocultures
CD19+CD27+IgD− B cells, CXCR5+CD57+CD25−CD4+ Tfh cells, and CXCR5+CD57+CD127−CD25+CD4+ Tfr cells were flow-sorted from iliac lymph nodes, and then cultured in 96-well U-bottom tissue culture plates (1 × 104 of each cell type per well) in the presence of T-cell activation and expansion beads (Miltenyi Biotec), as per manufacturer’s instructions. IgA secretion was determined after 5 days by enzyme-linked immunosorbent assay (ELISA).
IgA ELISA
Secretion of IgA was determined by Ig H-chain–specific ELISA. Nunc MaxiSorp flat-bottom 96-well plate (eBioscience) was coated with goat anti-human IgA (Jackson ImmunoResearch). Nonspecific binding sites were blocked with 1% bovine serum albumin/phosphate-buffered saline. Culture supernatants were added to the wells and incubated for 2 hours at 37°C. Plates were washed before adding horseradish peroxidase-conjugated donkey anti-human Ig H+L chain (Jackson ImmunoResearch). Bound IgA was visualized with TMB substrate (BioLegend).
Statistical analysis
Statistical analysis was performed using Prism software package using Mann-Whitney testing. Absolute cell counts for peripheral blood samples were calculated from hospital laboratory lymphocyte counts taken at the same time point as sampling. Absolute cell counts for lymph nodes were calculated from total cell count obtained at the time of processing the lymph node to form a single cell suspension.
Results
RTX effectively removes naïve circulating B cells but not memory B cells
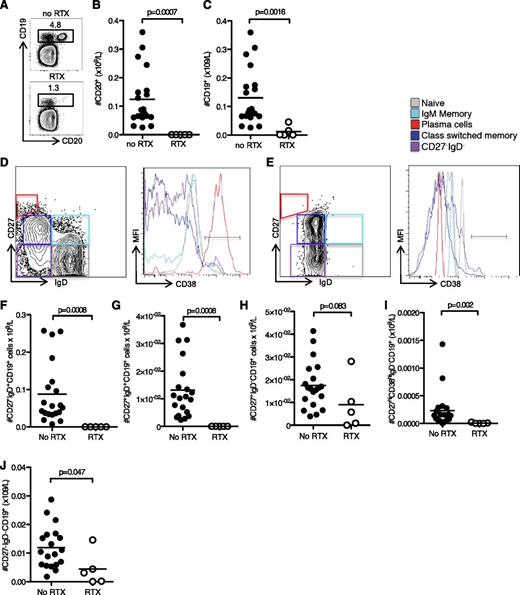
The impact of RTX on peripheral and tissue resident B cells has been of considerable interest because this B-cell–depleting therapy has been shown to be efficacious in treating hematologic malignancies and autoimmune diseases. Many studies have examined the effect of both single and multiple doses of RTX and shown variable removal of different B-cell subsets.36-40 In this study, we compared blood and lymph node samples from patients receiving a 500 mg dose of RTX 1 month prior to antibody incompatible kidney transplantation, with those from patients not receiving RTX. This single dose effectively removed CD20+ B cells within the peripheral blood, although a proportion of CD19+CD20− B cells were still detectable (Figure 1A-C). We characterized these cells in more detail using flow cytometry to divide them into IgD+CD27− naïve B cells, IgD+CD27+ memory cells, IgD−CD27+ memory cells, IgD−CD27− cells, and IgD−CD27hiCD38hi plasma cells. There were differences in these subsets between untreated patients (Figure 1D) and those who had received RTX (Figure 1E). In particular, we found that naïve IgD+ memory and plasma cells were significantly reduced in RTX-treated patients compared with control (Figure 1F-H). In contrast, IgD− memory and IgD−CD27− cells showed relative preservation (Figure 1I-J), consistent with previous reports.41-43 IgD−CD27− cells have previously been characterized as IgG−CD27− memory B cells,44 suggesting that the memory B-cell population in the peripheral blood is not as severely affected following RTX treatment as naïve B cells. Interestingly, these memory B-cell populations are reduced in the peripheral blood of patients with end-stage renal failure compared with healthy controls (see supplemental Figure 1 on the Blood Web site).
RTX effectively depletes naïve circulating B cells but not memory B cells. (A) Flow cytometric contour plots of CD19 and CD20, and numbers of CD20+ B cells, (B) CD19+ B cells, and (C) on peripheral blood lymphocytes taken from patients who have not (control patients, top) or have been (lower) treated with RTX. (D) Flow cytometric contour plot of naïve, IgD+ memory, IgD− memory, plasma cell, and CD27−IgD− subsets of CD19+ cells and MFI of CD38 on these subsets in a representative untreated patient. (E) Flow cytometric contour plot and MFI of CD38 of the same subsets in a representative RTX-treated patient. Bar graphs of (F) peripheral blood IgD+CD27−CD19+ naïve B cells, (G) IgD+CD27+CD19+ memory B cells, (H) CD38hiIgD−CD27hiCD19+ plasma cells, (I) IgD−CD27+CD19+ memory B cells, and (J) IgD−CD27−CD19+ B cells. For all bar graphs one symbol represents one individual, and the height of the bar represents the mean.
RTX effectively depletes naïve circulating B cells but not memory B cells. (A) Flow cytometric contour plots of CD19 and CD20, and numbers of CD20+ B cells, (B) CD19+ B cells, and (C) on peripheral blood lymphocytes taken from patients who have not (control patients, top) or have been (lower) treated with RTX. (D) Flow cytometric contour plot of naïve, IgD+ memory, IgD− memory, plasma cell, and CD27−IgD− subsets of CD19+ cells and MFI of CD38 on these subsets in a representative untreated patient. (E) Flow cytometric contour plot and MFI of CD38 of the same subsets in a representative RTX-treated patient. Bar graphs of (F) peripheral blood IgD+CD27−CD19+ naïve B cells, (G) IgD+CD27+CD19+ memory B cells, (H) CD38hiIgD−CD27hiCD19+ plasma cells, (I) IgD−CD27+CD19+ memory B cells, and (J) IgD−CD27−CD19+ B cells. For all bar graphs one symbol represents one individual, and the height of the bar represents the mean.
Tissue resident B cells are more resistant to removal by RTX
Flow cytometric analysis of lymphocytes from iliac lymph nodes showed no reduction in the number of CD19+CD20+ B cells in RTX-treated patients compared with controls (Figure 2A-C). This relative preservation of tissue B cells has been previously described.43 However, we noted a complete absence of CD38hiBcl6+ GC B cells in RTX-treated patients (Figure 2D-F). CD38hiBcl6+ GC B cells in untreated patients expressed higher levels of CD20 (Figure 2G-H) compared with CD38loBcl6− B cells, suggesting that they might be preferential targets for RTX-mediated removal. Having said that, whether this loss of GC B cells is due to direct depletion by RTX, or is indirect, and due for example to a failure of GC generation secondary to loss of a pre-GC B-cell population, cannot be determined from these data. These data show that RTX, in combination with tacrolimus and MMF, has no overall effect on B-cell numbers in the iliac lymph node, but GC B cells are not detectable following this treatment combination.
GC B cells are not detectable after RTX treatment. (A) Contour plots of CD19 and CD20 on lymphocytes from iliac lymph node of RTX-treated patients and controls. The number (B) and proportion (C) of CD20+ B cells in the lymph nodes of RTX-treated patients and controls. Contour plots (D), the percentage (E), and total number (F) of CD38+Bcl-6+CD19+ GC B cells in the lymph nodes of RTX-treated patients and controls. (G) Mean fluorescence intensity of CD20 on CD38−Bcl-6− non-GC B cells and CD38+Bcl-6+ GC B cells from control patients. (H) Mean fluorescence intensity of CD20 on CD19+ lymph node B cells from RTX-treated patients and controls. In bar graphs, one symbol represents one individual, and the height of the bar represents the mean.
GC B cells are not detectable after RTX treatment. (A) Contour plots of CD19 and CD20 on lymphocytes from iliac lymph node of RTX-treated patients and controls. The number (B) and proportion (C) of CD20+ B cells in the lymph nodes of RTX-treated patients and controls. Contour plots (D), the percentage (E), and total number (F) of CD38+Bcl-6+CD19+ GC B cells in the lymph nodes of RTX-treated patients and controls. (G) Mean fluorescence intensity of CD20 on CD38−Bcl-6− non-GC B cells and CD38+Bcl-6+ GC B cells from control patients. (H) Mean fluorescence intensity of CD20 on CD19+ lymph node B cells from RTX-treated patients and controls. In bar graphs, one symbol represents one individual, and the height of the bar represents the mean.
Memory B cells are preserved in the lymph nodes of RTX-treated patients
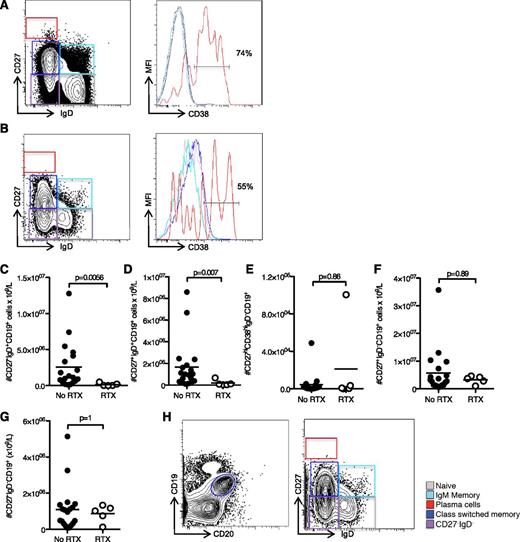
Despite the preservation of total B-cell number in the lymph node compared with peripheral blood, we assessed whether there was a change in the composition of the B-cell population following RTX treatment. We compared B-cell subsets in the lymph node between untreated (Figure 3A) and RTX-treated (Figure 3B) patients, as we had previously in the blood (see Figure 1). There was a significant reduction in IgD+CD27− naïve B cells and IgD+CD27+ memory cells in RTX-treated patients and controls (Figure 3C-D), together with preservation of IgD−CD27+ memory and IgD− CD27− cells in the lymph node (Figure 3E-F) of both patient groups. We did not find a reduction in IgD−CD27hiCD38hi plasma cells in the lymph node, consistent with their low expression of CD20 (Figure 3G). In addition, RTX-treated patients had an unusual population of CD19loCD20lo cells, which consisted largely of memory B cells (Figure 3H).
Characterization of CD19+ B cells in the lymph node suggests the cells resistant to removal are memory cells. Contour plots of cell subsets and MFI of CD38 on CD19+ cells from (A) iliac lymph nodes of controls, and (B) RTX-treated patients. Bar graphs of (C) lymph node CD27−IgD+CD19+ naïve B cells, (D) CD27+IgD+CD19+ memory B cells, (E) CD27hiCD38hiIgD−CD19+ plasma cells, (F) CD27+IgD−CD19+ memory B cells, and (G) CD27−IgD−CD19+ B cells. For all bar graphs, one symbol represents one individual, and the height of the bar represents the mean. (H) Detailed analysis of CD19loCD20lo cells alone in RTX-treated patients.
Characterization of CD19+ B cells in the lymph node suggests the cells resistant to removal are memory cells. Contour plots of cell subsets and MFI of CD38 on CD19+ cells from (A) iliac lymph nodes of controls, and (B) RTX-treated patients. Bar graphs of (C) lymph node CD27−IgD+CD19+ naïve B cells, (D) CD27+IgD+CD19+ memory B cells, (E) CD27hiCD38hiIgD−CD19+ plasma cells, (F) CD27+IgD−CD19+ memory B cells, and (G) CD27−IgD−CD19+ B cells. For all bar graphs, one symbol represents one individual, and the height of the bar represents the mean. (H) Detailed analysis of CD19loCD20lo cells alone in RTX-treated patients.
Lack of the GC after RTX treatment did not reduce Tfh within the lymph node
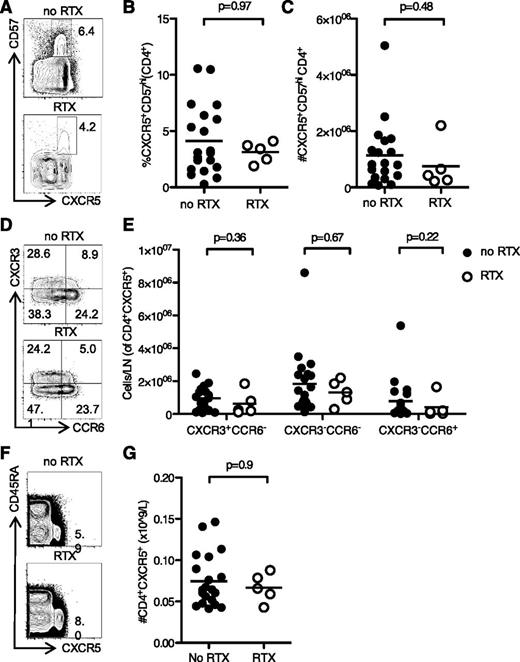
In mice, there is a positive correlation between the number of GC B cells and the number of Tfh cells,45 because Tfh maintenance requires sustained antigenic stimulation from GC B cells.22 Therefore, we were interested in whether the lack of GC B cells in RTX-treated patients reduced GC Tfh numbers. Although CXCR5 and ICOS are routinely used to identify T cells with B helper capacity, we were specifically interested in those T cells that would be located within the GC. CD57 has been shown to be a marker of T cells within the GC, and CXCR5+CD57+CD4+ T cells have been demonstrated to be Tfh in both phenotype and function.45 Surprisingly, there was no difference in the proportion or total number of CXCR5+CD57+CD4+ Tfh between RTX-treated patients and controls (Figure 4A-C). Tfh can be divided into Th1-like, Th2-like, and Th17-like subsets, each with a different capacity for B-cell help.29 To determine if loss of the GC affected these Tfh subsets differentially, we divided cells into CXCR3+CCR6−Th1-like Tfh, CXCR3−CCR6−Th2-like Tfh, and CXCR3−CCR6+Th17-like Tfh. We found no differences in these subsets in RTX-treated patients (Figure 4D-E). Furthermore, we did not see any alteration in the circulating CXCR5+CD4+ Tfh-like population in the blood of the same patients (Figure 4F-G). These data suggest that RTX treatment does not reduce GC Tfh in humans, despite completely eliminating GC B cells.
Loss of the GC after RTX treatment did not reduce Tfh numbers in the lymph node or peripheral blood. (A) Flow cytometric contour plots, (B) proportion, and (C) total number of CXCR5+CD57+CD4+ Tfh from iliac lymph nodes taken prior to kidney transplantation in RTX-treated patients and controls. (D) Contour plots, and (E) quantitation of CXCR3+CCR6−Th1-like Tfh (top left quadrant gate, panel D), CXCR3−CCR6−Th2-like Tfh (lower left quadrant gate, panel D), and CXCR3−CCR6+Th17-like lymph node Tfh cells (lower right quadrant gate, panel D). (F) Contour plots, and (G) bar graphs of peripheral blood CXCR5+CD4+ Tfh-like cells from the same patients in (A-E). In graphs, one symbol represents one individual, and the height of the bar represents the mean.
Loss of the GC after RTX treatment did not reduce Tfh numbers in the lymph node or peripheral blood. (A) Flow cytometric contour plots, (B) proportion, and (C) total number of CXCR5+CD57+CD4+ Tfh from iliac lymph nodes taken prior to kidney transplantation in RTX-treated patients and controls. (D) Contour plots, and (E) quantitation of CXCR3+CCR6−Th1-like Tfh (top left quadrant gate, panel D), CXCR3−CCR6−Th2-like Tfh (lower left quadrant gate, panel D), and CXCR3−CCR6+Th17-like lymph node Tfh cells (lower right quadrant gate, panel D). (F) Contour plots, and (G) bar graphs of peripheral blood CXCR5+CD4+ Tfh-like cells from the same patients in (A-E). In graphs, one symbol represents one individual, and the height of the bar represents the mean.
RTX did not alter Treg or Tfr cell numbers within the lymph node
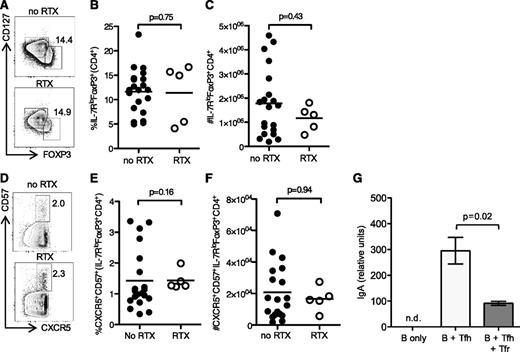
Suppressive Tfr cells have been previously described within the GC.11-13 These cells derive from Foxp3+ precursors, and in mice they are absolutely dependent on B cells and the GC for their formation.11,12,46 RTX has been described to alter proportions and function of Tregs in humans47,48 ; we, therefore, determined whether either the total Treg pool or the Tfr subset was altered after RTX treatment. There was no difference in FOXP3+CD127−CD4+ Treg in the lymph node (Figure 5A-C) or CXCR5+CD57+FOXP3+CD127−CD4+ Tfr cells (Figure 5D-F) between treated and untreated patients, suggesting that maintenance of the Tfr cell pool does not require an ongoing GC response in humans. To confirm that CXCR5+CD57+FOXP3+CD127−CD4+ Tfr cells function as suppressors of B-cell responses, we performed coculture assays of Tfh cells (CXCR5+CD57+CD25−CD4+) with memory B cells (CD27+IgD−CD19+) in the presence or absence of CXCR5+CD57+CD25+CD127−CD4+ Tfr cells. Tfr cells inhibited the production of T-dependent IgA by memory B cells after 5 days of culture (Figure 5G). Together, this data demonstrates that these cells have a surface phenotype and function consistent with Tfr cells, and they do not require an ongoing GC for their maintenance.
RTX did not alter Treg or Tfr numbers within the lymph node. (A) Flow cytometric contour plots, (B) quantitation of the proportion, and (C) total number of FOXP3+CD127−CD4+ Treg from iliac lymph nodes taken prior to kidney transplantation in RTX-treated patients and controls. (D) Contour plots, (E) proportion, and (F) total number of CXCR5+CD57+FOXP3+CD127−CD4+ Tfr from iliac lymph nodes. In graphs, one symbol represents one individual, and the height of the bar represents the mean. (G) IgA production in CD19+CD27+IgD− B cells cultured alone, coculture of B cells with CXCR5+CD57+CD25−CD4+ Tfh cells, or coculture of B cells with Tfh and CXCR5+CD57+CD127−CD25+CD4+ Tfr flow-sorted from iliac lymph nodes. Data in (G) are a representative biological replicate of 3 independent biological replicates. The height of the bars represent the mean of 4 technical replicates, and the error bars show the standard deviation. n.d., not detected above background.
RTX did not alter Treg or Tfr numbers within the lymph node. (A) Flow cytometric contour plots, (B) quantitation of the proportion, and (C) total number of FOXP3+CD127−CD4+ Treg from iliac lymph nodes taken prior to kidney transplantation in RTX-treated patients and controls. (D) Contour plots, (E) proportion, and (F) total number of CXCR5+CD57+FOXP3+CD127−CD4+ Tfr from iliac lymph nodes. In graphs, one symbol represents one individual, and the height of the bar represents the mean. (G) IgA production in CD19+CD27+IgD− B cells cultured alone, coculture of B cells with CXCR5+CD57+CD25−CD4+ Tfh cells, or coculture of B cells with Tfh and CXCR5+CD57+CD127−CD25+CD4+ Tfr flow-sorted from iliac lymph nodes. Data in (G) are a representative biological replicate of 3 independent biological replicates. The height of the bars represent the mean of 4 technical replicates, and the error bars show the standard deviation. n.d., not detected above background.
Discussion
In this study, we show that RTX treatment combined with tacrolimus and MMF led to a profound reduction in the numbers of circulating naïve and IgD+ memory B cells with relative preservation of IgD−CD27+ memory B cells, which has been well described in previous studies.43,49 The effects of RTX on tissue resident cells have been investigated, and it is clear that even a complete lack of circulating cells does not necessarily reflect deficiency of these cells in the tissues.49,50 In our cohort, RTX, in combination with tacrolimus and MMF, did not reduce the total B-cell numbers in the lymph node, but did result in the absence of CD38hiBcl6+CD19+ GC B cells and CD19+IgD+ naïve B cells, while leaving the CD27+CD19+ memory B-cell compartment intact. Although loss of GC B cells by this treatment has not been described in humans previously, it has been suggested that RTX can reduce the number of GC B cells in cynomolgus monkeys, based on an altered histologic appearance of follicles with a reduction in the number of CD19+ cells, rather than using specific GC markers.49
Consistent with previous reports showing variable but incomplete depletion of B cells in lymph nodes, we found no significant reduction in the number of B cells in the lymph node.40,43,50,51 However, we did find a significant reduction in the number of naïve and IgD+ memory cells in the lymph node, consistent with changes observed in the peripheral blood, although less profound. The residual cells in the lymph node showed a higher proportion of IgD−CD27+ memory B cells and IgD−CD27− memory B cells, which have been previously described as being resistant to removal by 1 dose of RTX.43 The mechanism by which memory cells are resistant to removal by 1 dose of RTX is unclear, although several have been postulated. B-cell depletion by RTX is dependent on antibody-dependent cellular cytotoxicity, thus anatomical variation in Fc-receptor expression may limit cell depletion, and an FcγRIIIa polymorphism has been associated with poor response to RTX in lymphoma patients.52 However, there is little evidence addressing which mechanisms predominate outside the setting of hematologic malignancies, where there is derangement of the normal environment, including depletion of complement and proportionately low numbers of effector cells due to the expansion of malignant cells, thus limiting antibody-dependent cellular cytotoxicity.52
We did not find a reduction in the number of plasma cells in the lymph node, which do not express CD20, and are therefore, not targeted by RTX. The absence of plasmablasts in the peripheral blood of RTX-treated patients may reflect the absence of an ongoing GC response and lack of plasmablast generation, as circulating plasma cells are short-lived outside of their bone marrow niche.53
The effects of RTX on T cells have been long debated, with suggestions that regulatory T-cell numbers and function may be increased following RTX treatment of autoimmune diseases,47,48,54 although other groups have described reductions in Treg numbers.37 Alterations in other subsets of CD4 T cells have also been suggested,47 however, despite these previous findings, and the absence of GC B cells in all RTX-treated patients, we found no alterations in Tfh or Tfr cell numbers. This observation is in contrast to what was observed in mice, where the depletion of GC B cells resulted in the loss of Tfh cells.22 However, data from patients with X-linked agammaglobulinemia, who have an almost complete arrest of B-cell differentiation in the bone marrow resulting in very few peripheral B cells, have a markedly reduced population of peripheral blood cTfh cells compared with healthy controls,55 consistent with a failure of formation, or maintenance, of Tfh in the absence of B cells in humans. Taken together with our observations, this suggests that, in humans, B cells are required for Tfh formation, but the GC is not necessary for their maintenance.
That ongoing interactions with GC B cells are dispensable for the persistence of GC T cells may occur because human Tfh and Tfr can be maintained without interactions with B cells, or that such interactions can be provided by non-GC B cells, such as the memory B cells we show persist despite RTX therapy. Either of these possibilities is intriguing, the latter because the maintenance of GC T cells by memory B cells has not been described in humans or mice previously. It has, however, been suggested that memory B cells are able to support lymph-node resident memory Tfh-like cells in mice,56,57 raising the possibility that the Tfh cells identified in this study may represent a lymphoid reservoir of memory Tfh cells similar to those described in mice,56 rather than a bona fide effector population. This seems unlikely, however, as CD57+CXCR5+CD4+ cells are prone to apoptosis,58 which is not a characteristic of persisting memory cells.
Alternatively, it is possible that our assessment at a single time point reflects repopulation of cells from cTfh-like cells, rather than persistence, despite an absence of GC cells. This would seem unlikely, however, as it would involve migration and de novo differentiation of lymph node Tfh- and Tfr-cell populations in the absence of a GC response.
Because of the absence of GC B cells and the reports that RTX can distort follicular architecture,49 we have used CD57 as a marker of GC-Tfh cells, in combination with the Tfh/Tfr surface receptor, CXCR5. Although the function of CD57 on Tfh cells is not well understood, it is clear that it identifies the GC-located Tfh cells.59 It has been shown that CXCR5+CD57− cells have similar B helper capacity to CXCR5+CD57+ cells in vitro and that ICOS expression is more important for the identification of cells that can support antibody production.60 CXCR5 and ICOS have therefore become the classical markers used to identify cells with effector Tfh function, which may reflect GC-Tfh and Tfh cells that act to support antibody production outside the follicle. It has also been shown that CXCR5+CD57+ cells are Tfh, located in the GC, and expressing ICOS and Bcl6, the key transcription factor for Tfh differentiation.45,61
Whether these cells are effector or memory populations, the preservation of these Tfh and Tfr cells following RTX, tacrolimus, and MMF treatment, is likely to result in the persistence of T cells that are able to support an allo- or autoreactive B-cell response once the B-cell pool recovers. Consistent with this, after a single dose of RTX to treat autoimmunity, disease flare and autoantibody production are common after repopulation of the B-cell pool.42,62,63 Together, our results suggest that additional treatment targeting Tfh may be required to reduce relapse risk following RTX treatment of autoimmune disease or in the setting of antibody incompatible transplantation.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This study was funded by a Wellcome Trust Program grant (083650/Z/07/Z) and a Lister Prize Fellowship to K.G.C.S. and supported by the National Institutes of Health Research Cambridge Biomedical Research Center. E.F.W. was supported by an Addenbrooke’s Charitable Trust research fellowship; M.A.L. was supported by a NHMRC Overseas Biomedical Fellowship, and the Biotechnology and Biological Sciences Research Council; and E.C.J. was supported by a Medical Research Council Clinical Research Fellowship, a Sackler Studentship and Evelyn Trust funding.
Authorship
Contribution: E.F.W. performed research, analyzed data, and wrote the paper; E.C.J. and O.S. performed research; J.A.B. provided help in establishing the study, obtained clinical samples, and reviewed the manuscript; D.R.W.J. provided help in establishing and undertaking this study and reviewed the manuscript; M.E. designed research and reviewed the manuscript; M.A.L. designed research, performed research, analyzed data, and wrote the paper; and K.G.C.S. designed research and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
The current affiliation for E.F.W. is Transplant Research Immunology Group, Nuffield Department of Surgical Sciences, University of Oxford, Oxford, United Kingdom; and for M.E. is Inserm UMR_S996/LabEx LERMIT, Clamart, France.
Correspondence: Michelle A. Linterman, Lymphocyte Signalling and Development, The Babraham Institute, Cambridge, United Kingdom; e-mail: michelle.linterman@babraham.ac.uk; and Kenneth G.C. Smith, Cambridge Institute for Medical Research and Department of Medicine, University of Cambridge School of Clinical Medicine, Cambridge, United Kingdom; e-mail: kgcs2@cam.ac.uk.
References
Author notes
M.A.L. and K.G.C.S. contributed equally to this study.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal