In this issue of Blood, Potts et al have identified a unique cell population in the yolk sac (YS) as the source of the first platelet-forming cells in mouse embryos. These cells are diploid and are produced via a pathway independent of hematopoietic progenitor cells (HPCs) generating polyploid megakaryocytes (MKs).1
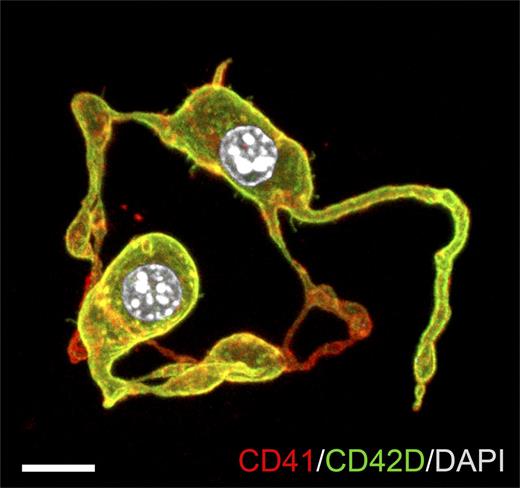
Representative confocal z-stack of diploid platelet-forming cells (DPFCs) showing proplatelet formation. E10.5 YS CD45−CD41high cells were cultured in serum-free medium with thrombopoietin for 72 hours. Cultures were stained with anti-CD41 and anti-CD42D antibodies. Nuclei were stained with 4,6-diamidino-2-phenylindole (DAPI). Scale bar represents 10 μm. See Figure 1H in the article by Potts et al that begins on page 2725.
Representative confocal z-stack of diploid platelet-forming cells (DPFCs) showing proplatelet formation. E10.5 YS CD45−CD41high cells were cultured in serum-free medium with thrombopoietin for 72 hours. Cultures were stained with anti-CD41 and anti-CD42D antibodies. Nuclei were stained with 4,6-diamidino-2-phenylindole (DAPI). Scale bar represents 10 μm. See Figure 1H in the article by Potts et al that begins on page 2725.
When and how hematopoiesis emerges and proceeds are still intriguing questions. Despite remarkable advances in this field,2 details of blood cell development are still largely veiled in mystery. Understanding the mechanism by which hematopoietic cells are generated is critical and could lead to novel strategies for the making of functional hematopoietic cells in vitro for regenerative medicine using not only cord blood hematopoietic stem and progenitor cells but also pluripotent stem cells such as embryonic stem (ES) and induced pluripotent stem (iPS) cells.3,4
Mature MKs derived from HPCs, which are characterized by polyploid nuclei, have been thought to generate platelets from the beginning throughout life. However, Potts et al have discovered previously unrecognized diploid cells in the YS as the first platelet-forming cells, which are distinct from polyploid MKs. These unexpected findings provide novel insights into our understanding of the development of platelet-producing cells and therapeutic control of them.
Primitive hematopoiesis occurs in the YS on embryonic day (E) 7 in mice. First MK progenitors, which are immunophenotypically CD45+CD41low, could be detected as early as E7.5 in the YS.5 These progenitors are derived from conventional HPCs and differentiate into mature polyploid MKs, which undergo proplatelet formation. Because MKs are first detected at E8.5 to E9.5 YS,5,6 these MK progenitors have been thought to mature rapidly and give rise to platelets until definitive hematopoiesis begins to produce platelets. However, Potts et al found that these conventionally identified MK progenitors on as early as E8.5 YS do not mature into proplatelet-forming cells within 72 hours in culture. They also found that platelets are released into the bloodstream as early as E9.5, then dramatically increase in number by E10.5.1 Based on these findings, they hypothesized that an alternative cell population is responsible for initial platelet formation.
To this end, the investigators compared the transcriptional profiles of E10.5 YS cell fractions with E13.5 fetal liver reference fractions. Unexpectedly, the profile of previously uncharacterized CD45−CD41high cells in the YS most resembled that of E13.5 fetal liver MKs. This population indeed expressed MK-associated proteins including myeloproliferative leukemia protein, CD42D (glycoprotein V), and acetylcholinesterase. Although the majority of CD45−CD41high cells remained diploid and did not exhibit the high ploidy range associated with MKs, half of them formed proplatelets within 72 hours in culture (see figure) and, surprisingly, also did so in vivo while in a diploid state. They therefore defined this fraction as DPFCs.
CD41+ cells in the YS at E7.75 to E8.25 express vascular endothelial cadherin (VECAD) and then by E8.5 diverge into VECAD+ and VECAD− fractions. Potts et al clarified that at E8.5 VECAD+CD41high cells contain all HPC progeny (myeloid/erythroid and MK progenitors), whereas VECAD−CD41high cells contain immature DPFC precursors (pre-DPFCs) capable of acute proplatelet formation. They finally performed proplatelet formation assays using Runx1-null YS cells, in which HPC formation is completely blocked7 to address the next question of whether HPCs and DPFCs both progress via VECAD-positive precursors, that is, if HPCs give rise to DPFCs. VECAD−CD41high pre-DPFCs capable of generating proplatelets in vitro were detected even in Runx1-null E8.5 YS. Furthermore, although fewer than wild type, there existed pre-DPFCs and DPFCs producing proplatelets in the E8.5 and E10.5 Runx1Δ/Δ YS, respectively. All these data summarily suggested that DPFCs develop independently of HPCs.
Now, newly identified DPFCs have been proved to play a role in hematopoietic development. DPFCs are quite unique in their rapid terminal differentiation, which is achieved by skipping endomitosis, a critical process for MKs to increase ploidy. Given the requirement of acute platelet production to supply platelets timely upon initiation of embryonic circulation, rapid platelet production at the expense of the amount of platelet production per single producer cell seems reasonable. However, we do not know when and how HPCs and DPFCs diverge and how the platelet production by DPFCs is regulated. The investigators showed that DPFCs produce platelets in a thrombopoietin-independent manner. What are the alternative regulators? Furthermore, when do conventional MKs take over platelet production instead of DPFCs in fetus? Many exciting questions remain to be answered. Future studies will undoubtedly deepen our understanding of embryonic hematopoiesis, especially the mechanisms of how the first hematopoiesis arises.
Finally, what kind of impact do DPFCs have on ex vivo expansion of platelets? Extensive efforts have been taken to improve the efficiency of platelet production, particularly from ES/iPS cells.3,4,8 One of the rate-limiting steps on ex vivo generation of platelets is MK maturation. The degree of MK polyploidization is correlated with the amount of platelet production. However, hematopoietic progenies derived from ES/iPS cells largely exhibit characters of primitive but not definitive blood cells and often recapitulate YS hematopoiesis. It will therefore be very interesting to examine whether platelet production by ES/iPS cells follows the DPFC pathway or HPC-derived MK pathway or both switching in a time-dependent manner. These approaches should give a hint to improve the therapeutic manipulation of ES/iPS cells for platelet production.
Conflict-of-interest disclosure: The authors declare no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal