In this issue of Blood, Palladini and colleagues address incidence of and risk factors for end-stage renal disease (ESRD) and proffer a new renal response system for patients with amyloid light-chain (AL) amyloidosis using a test set of 461 patients from Pavia, Italy, and a validation set of 271 patients from Heidelberg, Germany.1 Validation of organ response criteria has trailed behind the progress made in the field of AL amyloidosis in diagnosis, prognosis, and hematologic response,2-6 but Palladini and colleagues’ study helps breach this gap.
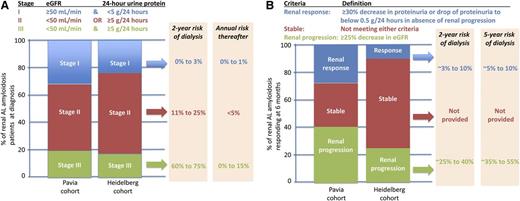
Distilling the frequencies of and outcomes associated with renal stage (A) and suggested renal response (B). Data aggregated from the article by Palladini et al that begins on page 2325.1
Distilling the frequencies of and outcomes associated with renal stage (A) and suggested renal response (B). Data aggregated from the article by Palladini et al that begins on page 2325.1
The current study demonstrates that most cases of ESRD in patients with AL amyloidosis of the kidney occur in the first 2 years, with 17% to 30% of surviving renal patients on dialysis by that time. Thereafter, the risk of dialysis is only a few percent per year. These findings are comparable to those of a British group’s analysis of 752 patients with AL amyloidosis involving the kidney, ie, that 13% experience progression to ESRD at a median time of 26.8 months.7
The renal staging system formulated by Palladini and colleagues goes further and separates the 23% to 32% of patients who have virtually no risk of ESRD, the 50% to 60% of patients with an 11% to 25% risk of dialysis at 2 years, and the 17% to 20% of renal amyloid patients who have a 60% to 75% risk of dialysis at 2 years (see figure, panel A) by dichotomizing patients into those with less than 5 g/24-hour urinary protein excretion and those with an estimated glomerular filtration rate (eGFR) of ≥50 mL/min/1.73 m2 and then classifying them as neither adverse (stage I), either adverse (stage II), or both adverse (stage III).
Their work on renal response is also exceptional but potentially more controversial. By using receiver operating characteristic (ROC) analyses of changes of variables from baseline to 6 months and looking for those that predict progression to dialysis at 24 months, they define renal response as a decrease in 24-hour total urinary protein excretion by ≥30% (or urinary proteinuria to <0.5 g/24 hours) and renal progression as a decrease of eGFR of ≥25% (see figure, panel B). They also demonstrate the importance of lowering the serum free light chain to protect patients from ESRD.
Their definitions of renal response and progression focus on “renal mortality” (ie, ESRD) rather than “renal morbidity.” As compared with the 2005 consensus guidelines,8 their definition of renal response allows for a 30% (rather than a 50%) reduction in proteinuria. Their focus on a 6-month time point is conducive to clinical trial assessment, which is an advantage. However, the morbidity of renal involvement is not restricted to the minority of patients who develop ESRD; rather, it relates to the low serum albumin that typically accompanies proteinuria and causes edema that may be mild or severe, the latter of which results in anasarca, effusions, worsening congestive heart failure, and orthostasis from intravascular depletion. For renal response, benchmarking to something in addition to ESRD may be more appropriate, because the absence of harm is not the same as the presence of benefit. The authors’ position of not linking renal response to overall survival, which is dissimilar to the approach taken by Leung et al,9 is not unreasonable. However, normalization of serum albumin at 2 years or 95% reduction of proteinuria at 2 years could be potential targets for ROC analysis to refine response criteria.
The authors’ definition of renal progression retains and validates the 20058 eGFR criterion but excludes the proteinuria criterion (a 50% increase in proteinuria of at least 1 g/24 hours), because the 50% increase in proteinuria in the absence of eGFR deterioration provided less discrimination in renal outcome than did eGFR.
The authors should be congratulated on pushing the amyloidosis community to readdress renal response, for validating a part of the currently accepted renal progression criteria, and for emphasizing the need to have valid measures at reasonable time points (ie, 6 months rather than the time-unrestricted “best response”). Let us keep the field moving forward!
Conflict-of-interest disclosure: The author declares no competing financial interests.