Key Points
HCMV infection in early life is associated with rapid phenotypic and functional differentiation of NK cells.
Emergence of CD57+ NK cells is attenuated in children lacking NKG2C.
Abstract
Natural killer (NK) cells differentiate and mature during the human life course; human cytomegalovirus (HCMV) infection is a known driver of this process. We have explored human NK cell phenotypic and functional maturation in a rural African (Gambian) population with a high prevalence of HCMV. The effect of age on the frequency, absolute number, phenotype, and functional capacity of NK cells was monitored in 191 individuals aged from 1 to 49 years. Increasing frequencies of NK cells with age were associated with increased proportions of CD56dim cells expressing the differentiation marker CD57 and expansion of the NKG2C+ subset. Frequencies of NK cells responding to exogenous cytokines declined with age in line with a decreased proportion of CD57− cells. These changes coincided with a highly significant drop in anti-HCMV IgG titers by the age of 10 years, suggesting that HCMV infection is brought under control as NK cells differentiate (or vice versa). Deletion at the NKG2C locus was associated with a gene dose-dependent reduction in proportions of CD94+ and CD57+ NK cells. Importantly, anti-HCMV IgG titers were significantly elevated in NKG2C−/− children, suggesting that lack of expression of NKG2C may be associated with altered control of HCMV in childhood.
Introduction
Natural killer (NK) cells play essential roles in controlling infection and surveillance for damaged, dysfunctional, or neoplastic cells.1 NK cells differentiate during the human life course. CD56bright cells are the least-differentiated population of peripheral blood NK cells, expressing c-kit and high levels of the c-type lectin-like receptor CD94/NKG2A, CD62L, and natural cytotoxicity receptors (NCRs) NKp30 and NKp46, and lacking expression of killer cell immunoglobulin-like receptors (KIR), CD16, and CD57.2-5 CD56bright NK cells express cytokine receptors and produce interferon (IFN)-γ in response to cytokines. In contrast, CD56dim cells express FcγRIII(CD16); express varying levels of CD94/NKG2A, KIR, NCRs, and perforin; retain their ability to secrete IFN-γ; and have higher cytotoxic capacity.3 Heterogeneity within the CD56dim subset is associated with acquisition of CD57.2,4,5 CD56dimCD57− NK cells are phenotypically and functionally similar to CD56bright cells, whereas CD56dimCD57+ cells produce little IFN-γ and have shorter telomeres and lower proliferative capacity,5,6 but degranulate extensively after crosslinking of CD16.2,5 Acquisition of CD57 is associated with onset of expression of NKG2C, although the codependence of these events and their implications for function are not understood.7,8
Although the external drivers of NK cell differentiation are incompletely understood, inflammation, associated with infection or loss of immune homeostasis, plays a key role.9 This view is supported by evidence that the late differentiation marker, CD57, can be induced on NK cells by high concentrations of IL-2,5 that NKG2C+ NK cells can be expanded by coculture with human cytomegalovirus (HCMV)-infected fibroblasts,10 that HCMV-seropositive individuals have increased frequencies of NKG2C+ NK cells,10-13 and that there is rapid expansion of CD57+NKG2Chi NK cells during acute HCMV infection14 and in individuals infected with Epstein Barr virus (EBV),7 hantavirus,15 hepatitis viruses,16 and chikungunya virus.17
Among Caucasians, NK cell maturation is highly age-dependent. Marked phenotypic and functional differences are observed between NK populations in cord blood, in young children, in adults, and in elderly individuals.18-22 Young children have higher frequencies of CD56brightCD16− and NKG2A+NKG2C− NK cells compared with adults, and younger adults have higher frequencies of these cells compared with the elderly.18-22 Moreover, NCR+ and NKG2D+ NK cells decrease in frequency with increasing age, concomitant with loss of CD62L and acquisition of CD57.2,4,18,22 NK cell cytokine production decreases with increasing age, but cytotoxic responses are conserved.9,20,23 There is, however, a lack of data from older children and teenagers.
The extent to which NK cell differentiation is explained by either aging, per se, or by cumulative exposure to infection is unclear. Among allogeneic hematopoietic stem cell transplant recipients, the first wave of repopulating NK cells comprises predominantly CD56bright or CD56dimCD94+cells; KIR+ and CD57+ cells can take up to 1 year to emerge.2,24 However, among patients who reactivate HCMV after transplantation, NKG2C+CD57+ NK cells can be detected within 3 months, and the host’s pretransplantation repertoire is fully reconstituted within 6 months, suggesting that exposure to infection is a significant determinant of NK cell maturation rates.24-26
Together, these data suggest that age-related changes in NK cell phenotype and function may be modified by the infection status of the host and that rates of change across populations may depend on the prevalence of particular infections. If so, the prevalence of infections such as HCMV may have far-reaching implications for risk for other infections, cancers, or autoimmune disease. To begin to address this important aspect of NK cell biology, we have characterized NK cell phenotype and function in an African population that is itself characterized by a high burden of infectious disease, including near-universal HCMV infection.
Materials and methods
Study subjects
This study was approved by the ethical review committees of the Gambia Government/Medical Research Council and the London School of Hygiene and Tropical Medicine. Participants were recruited from the villages of Keneba, Manduar, and Kantong Kunda in the West Kiang district, The Gambia. After fully informed consent was obtained in accordance with the Declaration of Helsinki, including parental/guardian consent for minors, venous blood samples were collected from 191 individuals aged 1 to 49 years. Individuals with signs or symptoms of current disease or who were known to be pregnant or infected with HIV were excluded. Plasma was screened for IgG against HCMV(BioKit), tetanus toxoid (Holzel Diagnostica), hepatitis B surface antigen (Diasorin), and EBV nuclear antigen (Euroimmun). Subject characteristics are shown in Table 1.
Cohort characteristics
| Age group, years . | n (male/female) . | HCMV IgG+, n (%) . | HCMV IgG titer, IU/mL, median (range) . | EBV nuclear antigen IgG+, n (%) . | EBV nuclear antigen IgG titer, IU/mL, median (range) . | NKG2C genotype, n (%)* . | ||
|---|---|---|---|---|---|---|---|---|
| +/+ . | +/− . | −/− . | ||||||
| 1-2 | 23 (9/14) | 20 (86.9) | 487.5 (81.8-845.2)† | 12 (52.2) | 107.0 (48.5-178.6) | 11 (47.8) | 10 (43.4) | 2 (8.7) |
| 3-5 | 19 (6/13) | 18 (94.7) | 288.4 (80.9-1681.8) | 13 (68.4) | 134.0‡ (32.5-328.7) | 7 (37.8) | 10 (52.6) | 2 (10.5) |
| 6-9 | 18 (11/7) | 18 (100) | 361.1 (89.2-2200.2)¶ | 16 (88.9) | 103.6 (33.1-219.7) | 8 (47.0) | 7 (41.2) | 2 (11.8) |
| 10-12 | 20 (10/10) | 20 (100) | 215.4 (43.4-1693.6) | 18 (90.0) | 119.3§ (37.2-359.5) | 8 (44.4) | 8 (44.4) | 2 (11.1) |
| 13-15 | 23 (10/13) | 23 (100) | 252.6 (51.5 −1057.9) | 16 (70.0) | 114.6 (29.7-193.4) | 11 (47.8) | 10 (43.4) | 2 (8.7) |
| 16-19 | 23 (11/12) | 23 (100) | 177.6 (61.2-678.1) | 18 (78.2) | 99.9 (23.9-195.2) | 10 (47.6) | 8 (38.1) | 3 (14.3) |
| 20-25 | 22 (11/11) | 22 (100) | 252.5 (81.5-828.4) | 19 (86.4) | 93.9 (27.6-171.7) | 11 (52.4) | 8 (38.1) | 2 (9.5) |
| 26-39 | 22 (13/9) | 22 (100) | 165.9 (39.0-968.4) | 19 (86.4) | 88.8 (24.9-272.7) | 14 (73.7) | 3 (15.7) | 2 (10.5) |
| 40-49 | 21 (10/11) | 21 (100) | 191.2 (53.5-735.2) | 13 (61.9) | 73.4 (24.0-183.2) | 14 (70.0) | 4 (20.0) | 2 (10.0) |
| Total | 191 (91/100) | 187 (97.9) | 252.6 (39-2200.1) | 145 (75.9) | 101.8 (23.9-359.5) | 94 (51.9) | 68 (37.6) | 19 (10.5) |
| Age group, years . | n (male/female) . | HCMV IgG+, n (%) . | HCMV IgG titer, IU/mL, median (range) . | EBV nuclear antigen IgG+, n (%) . | EBV nuclear antigen IgG titer, IU/mL, median (range) . | NKG2C genotype, n (%)* . | ||
|---|---|---|---|---|---|---|---|---|
| +/+ . | +/− . | −/− . | ||||||
| 1-2 | 23 (9/14) | 20 (86.9) | 487.5 (81.8-845.2)† | 12 (52.2) | 107.0 (48.5-178.6) | 11 (47.8) | 10 (43.4) | 2 (8.7) |
| 3-5 | 19 (6/13) | 18 (94.7) | 288.4 (80.9-1681.8) | 13 (68.4) | 134.0‡ (32.5-328.7) | 7 (37.8) | 10 (52.6) | 2 (10.5) |
| 6-9 | 18 (11/7) | 18 (100) | 361.1 (89.2-2200.2)¶ | 16 (88.9) | 103.6 (33.1-219.7) | 8 (47.0) | 7 (41.2) | 2 (11.8) |
| 10-12 | 20 (10/10) | 20 (100) | 215.4 (43.4-1693.6) | 18 (90.0) | 119.3§ (37.2-359.5) | 8 (44.4) | 8 (44.4) | 2 (11.1) |
| 13-15 | 23 (10/13) | 23 (100) | 252.6 (51.5 −1057.9) | 16 (70.0) | 114.6 (29.7-193.4) | 11 (47.8) | 10 (43.4) | 2 (8.7) |
| 16-19 | 23 (11/12) | 23 (100) | 177.6 (61.2-678.1) | 18 (78.2) | 99.9 (23.9-195.2) | 10 (47.6) | 8 (38.1) | 3 (14.3) |
| 20-25 | 22 (11/11) | 22 (100) | 252.5 (81.5-828.4) | 19 (86.4) | 93.9 (27.6-171.7) | 11 (52.4) | 8 (38.1) | 2 (9.5) |
| 26-39 | 22 (13/9) | 22 (100) | 165.9 (39.0-968.4) | 19 (86.4) | 88.8 (24.9-272.7) | 14 (73.7) | 3 (15.7) | 2 (10.5) |
| 40-49 | 21 (10/11) | 21 (100) | 191.2 (53.5-735.2) | 13 (61.9) | 73.4 (24.0-183.2) | 14 (70.0) | 4 (20.0) | 2 (10.0) |
| Total | 191 (91/100) | 187 (97.9) | 252.6 (39-2200.1) | 145 (75.9) | 101.8 (23.9-359.5) | 94 (51.9) | 68 (37.6) | 19 (10.5) |
NKG2C genotypes were obtained from a total of 181 individuals.
Significantly higher anti-HCMV IgG titers compared with 16- to 19-year-olds and all groups older than 26 years; P < .05, analysis of variance.
Significantly elevated anti-EBV nuclear antigen IgG titers compared with all groups older than 16 years.
Significantly higher anti-HCMV IgG titers compared with all groups older than 16 years; P < 0.01, analysis of variance.
Significantly elevated anti-EBV nuclear antigen IgG titers compared with all groups older than 20 years.
Peripheral blood mononuclear cell preparation and culture
Peripheral blood mononuclear cells (PBMCs) were isolated by density gradient centrifugation (Histopaque, Sigma) and analyzed ex vivo and after 18-hour culture with cytokines (5 ng/mL rhIL-12; Peprotec) plus 50 ng/mL rhIL-18 (R&D Systems) or with K562 cells (an Effector:Target ratio of 2:1). Fluorescein isothiocyanate (FITC)-conjugated anti-CD107a (BD Biosciences) was added throughout the culture. Brefeldin A and Monensin (BD Biosciences) were added after 15 hours.
Flow cytometry
PBMCs were incubated with combinations of the following monoclonal antibodies: anti-CD3-V500, anti-CD56-PeCy7 and anti-CD94-FITC, anti-NKG2C-PE and anti-NKG2A-APC, anti-CD8-PeCy7, anti-CD57-e450 and anti-CD16-APC-e780 or APC, anti-CD4-PE and anti-CD45RA-APC-H7, anti-CD8-PeCy7, anti-CD27-FITC, anti-CD28-PeCy7 and anti-CCR7-APC, anti-CD45-FITC, anti-CD11c-PE, anti-CD19-PeCy5, anti-CD123-efluor450 and anti-CD14-APCe780, anti-CD107a-FITC, anti-CD25PE, and anti-IFN-γ-APC-efluor780 (supplemental Methods, available on the Blood Web site). Cells were acquired on a LSRII® flow cytometer using FacsDiva® software. Data analysis was performed using FlowJo® (TreeStar).
NKG2C genotyping
DNA was extracted from whole blood (Wizard genomic DNA extraction kit, Promega), and the NKG2C genotype was determined by touchdown PCR (Phusion® High Fidelity PCR kits, New England Biolabs).27 PCR primers and conditions are described in the supplemental Methods.
Statistical analysis
Statistical analysis was performed using Statview and Stata version 13.1. Nonlinear effects of age were modeled using natural cubic splines in linear regression models; P values (F-test) and R2 values were obtained from these models. One-way analysis of variance was used to compare responses of individuals of different genotypes. Differences between NK cell subsets were compared using Wilcoxon signed rank tests.
Results
High rates of HCMV and EBV infection in the study population
HCMV infection rates are high in Africa, and thus, as expected, only 4 of the 191 individuals were HCMV-seronegative; seronegative patients were aged between 1 and 3 years, suggesting universal HCMV infection within the first 3 years of life (Table 1). Interestingly, anti-HCMV antibody titers were significantly higher among those younger than 10 years than in older individuals, suggesting that optimal control of HCMV infection takes some years to develop (Table 1). EBV infection was also common, with 75% of the cohort being seropositive for EBV nuclear antigen. EBV nuclear antigen seropositivity rates were lowest in children 2 years old or younger, and anti-EBV nuclear antigen titers tended to be higher in those younger than 15 years than in older individuals (Table 1).
NK cell numbers and frequencies change with age
NK cell numbers and frequencies and the distribution of CD56bright and CD56dim subsets (Figure 1A) were analyzed by age group (Figure 1). Consistent with previous observations,18-22 the proportion of NK cells among peripheral blood lymphocytes increased significantly with age, reaching a plateau at approximately 15 years (Figure 1B). Within the total NK cell population, the proportion of CD56bright NK cells declined significantly with increasing age (Figure 1C), and the proportion of CD56dim cells increased (Figure 1D), with subset distribution stabilizing at approximately 10 to 12 years (supplemental Figure 1). The absolute number of peripheral blood CD56bright and CD56dim NK cells declined with age, indicating that the increased frequency of CD56dim cells in older individuals was not sufficient to offset the overall decline in NK cell numbers (supplemental Figure 1; supplemental Table 1).
Age-related changes in NK cell frequencies (A). NK cells were identified within PBMC after gating on singlets and viable lymphocytes. CD56+CD3−NK cells were then subsequently gated into CD56bright and CD56dim subsets. Frequencies of (B) all NK cells, (C) CD56bright, and (D) CD56dim NK cells are shown for each age group. Horizontal bars represent median values, boxes extend from the 25th to the 75th percentile, and whiskers represent the 95th percentiles. Asterisks represent significant trends across the entire cohort (*P < .05, ***P < .001, F-test).
Age-related changes in NK cell frequencies (A). NK cells were identified within PBMC after gating on singlets and viable lymphocytes. CD56+CD3−NK cells were then subsequently gated into CD56bright and CD56dim subsets. Frequencies of (B) all NK cells, (C) CD56bright, and (D) CD56dim NK cells are shown for each age group. Horizontal bars represent median values, boxes extend from the 25th to the 75th percentile, and whiskers represent the 95th percentiles. Asterisks represent significant trends across the entire cohort (*P < .05, ***P < .001, F-test).
These early and very marked changes in NK cell phenotype contrasted with more gradual changes in T-cell phenotype (supplemental Figure 2). Consistent with previous studies,28,29 we observed a steady decline in naive CD4+ and CD8+ T-cell frequencies, with a parallel increase in frequencies of effector memory and central memory T cells. However, in contrast to published data,28,29 the frequency of terminally differentiated effector memory (TEMRA) cells was already high in young children and did not increase further with age, possibly reflecting high levels of antigen exposure in early life. A decline in absolute numbers of all myeloid and lymphoid cell populations was observed throughout life (supplemental Tables 2 and 3A-B).
Phenotypic differentiation of NK cells is biphasic and is most rapid during the first 5 years of life
We identified 3 distinct populations of CD56dim NK cells: CD57−, CD57+, and those with intermediate CD57 expression (CD57int)6 (Figure 2A). The proportion of CD57− CD56dim NK cells declined significantly with age, mirrored by increasing proportions of CD57+ NK cells; the proportion of CD57int cells was stable, consistent with this being a transitional population (Figure 2B). Strikingly, this was a biphasic rather than a linear process, with the most marked changes in CD57 subset distribution occurring in children aged 5 years or younger, with very little change in subset distribution after the age of 10 years (supplemental Figure 3A-C).
Age-related changes in frequencies of CD57- and c-type lectin-like receptor- expressing NK cell subsets (A). CD56dim cells were gated into CD57−, CD57intermediate, and CD57+ subsets. The CD57− population was gated using an isotype-matched control reagent, and the CD57+ gate was set at an MFI of 3000. (B) Frequency distribution by age group of CD57−, CD57int, and CD57+ subsets within the CD56dim NK cell population. Asterisks denote statistically significant trends for changes in NK cell subset frequency by age (***P < .001, F-test). (C) Gating strategy for CD94+ cells and (E) CD94 NKG2A+ and CD94+NKG2C+ cells within the CD56dim NK cell subset. (D) Frequencies of CD94+ and (F) NKG2A+, and NKG2C+ NK cells by age group. Asterisks denote statistically significant differences in frequencies of NKG2A+ and NKG2C+ cells by age group (*P < .05, analysis of variance). Horizontal bars represent median values, boxes extend from the 25th to the 75th percentile, and whiskers represent the 95th percentiles. Age groups are as shown in Figure 1.
Age-related changes in frequencies of CD57- and c-type lectin-like receptor- expressing NK cell subsets (A). CD56dim cells were gated into CD57−, CD57intermediate, and CD57+ subsets. The CD57− population was gated using an isotype-matched control reagent, and the CD57+ gate was set at an MFI of 3000. (B) Frequency distribution by age group of CD57−, CD57int, and CD57+ subsets within the CD56dim NK cell population. Asterisks denote statistically significant trends for changes in NK cell subset frequency by age (***P < .001, F-test). (C) Gating strategy for CD94+ cells and (E) CD94 NKG2A+ and CD94+NKG2C+ cells within the CD56dim NK cell subset. (D) Frequencies of CD94+ and (F) NKG2A+, and NKG2C+ NK cells by age group. Asterisks denote statistically significant differences in frequencies of NKG2A+ and NKG2C+ cells by age group (*P < .05, analysis of variance). Horizontal bars represent median values, boxes extend from the 25th to the 75th percentile, and whiskers represent the 95th percentiles. Age groups are as shown in Figure 1.
The frequency of NK cells expressing CD94, which partners both NKG2A and NKG2C at the cell surface, remained stable throughout life, suggesting that the proportion of NK cells expressing either NKG2A or NKG2C also remains stable (Figure 2C-F; supplemental Figure 3D). However, within the CD94+ population, the proportion of NKG2A+ cells decreased with increasing age (Figure 2E; P = .03, analysis of variance), whereas the proportion of NKG2C+ cells increased (Figure 2F; P = .02, analysis of variance). Increasing proportions of NKG2C+ NK cells were offset by decreasing NK cell numbers, such that the absolute number of NKG2C+ cells remained stable throughout life (supplemental Table 1).
We then assessed whether changes in CD57 expression mirrored changes in NKG2A/NKG2C expression (Figure 3). The proportion of CD57− cells within the NKG2A+ subset decreased significantly with increasing age, with a reciprocal enrichment of CD57int and CD57+ NK cells (Figure 3A). Nevertheless, the majority of NKG2A+ NK cells remained CD57−, even in older individuals (Figure 3A). In contrast, NKG2C+ NK cells are frequently CD57+ even in children younger than 2 years, and the majority of NKG2C+ NK cells are CD57+ by the age of 5 years (Figure 3B). The mean fluorescence intensity (MFI) of CD57 expression was very low on NKG2A+ NK cells (at all ages) but increased significantly with increasing age on NKG2C+ cells (Figure 3C; supplemental Figure 3G-H), suggesting that NKG2C+ NK cells differentiate rapidly in this cohort (gaining full CD57 expression very early in life), whereas NKG2A+ NK cells differentiate only very slowly. This rapid expansion and differentiation of the NKG2C+ NK cell population is likely a consequence of perinatal HCMV infection. Moreover, anti-HCMV IgG titer was negatively correlated with the frequency of CD57+ NK cells (supplemental Figure 4), suggesting that advanced NK cell differentiation may be associated with control of HCMV or vice versa. EBV serostatus, which has been associated with altered NK cell phenotype in HCMV-exposed Europeans,7 had no significant effect on NK cell subset distribution, other than a minor increase in CD56dim cell frequency (supplemental Figure 5A-G), supporting a recent paper suggesting that acute EBV coinfection has no major effect on NKG2C+CD57+NK cells.30
CD57 is preferentially expressed on NKG2C+ NK cells. CD56dim NK cells were gated as in Figure 1A, and the frequency of CD57−, CD57int, and CD57+ cells is shown within (A) CD94/NKG2A+ or (B) CD94/NKG2C+ NK cells, by age group. (C) MFI for CD57 expression on NKG2A+ and NKG2C+ NK cells by age group. Horizontal bars represent median values, boxes extend from the 25th to the 75th percentile, and whiskers represent the 95th percentiles. Asterisks denote statistically significant trends by age within each subset (**P < .01; ***P < .001, F-test). Age groups are as shown in Figure 1.
CD57 is preferentially expressed on NKG2C+ NK cells. CD56dim NK cells were gated as in Figure 1A, and the frequency of CD57−, CD57int, and CD57+ cells is shown within (A) CD94/NKG2A+ or (B) CD94/NKG2C+ NK cells, by age group. (C) MFI for CD57 expression on NKG2A+ and NKG2C+ NK cells by age group. Horizontal bars represent median values, boxes extend from the 25th to the 75th percentile, and whiskers represent the 95th percentiles. Asterisks denote statistically significant trends by age within each subset (**P < .01; ***P < .001, F-test). Age groups are as shown in Figure 1.
Rapid functional maturation of NK cells during childhood in The Gambia
To assess the functional consequences of these phenotypic changes, PBMCs were cultured in vitro with K562 target cells or with high concentrations of cytokines (IL-12 and IL-18; HCC); NK cell degranulation (CD107a), CD25, and IFN-γ expression were assessed by flow cytometry (Figure 4). Spontaneous low-level degranulation and IFN-γ production was observed among unstimulated cells from children younger than 10 years, perhaps indicating in vivo NK cell activation (Figure 4C,I). Incubation with K562 cells increased NK cell degranulation, but this did not differ with age (Figure 4D). Conversely, degranulation and upregulation of CD25 and IFN-γ production in response to HCC (Figure 4E,H,K) were both strongly age-related, being significantly higher in children younger than 10 years than in older individuals (supplemental Figure 6).
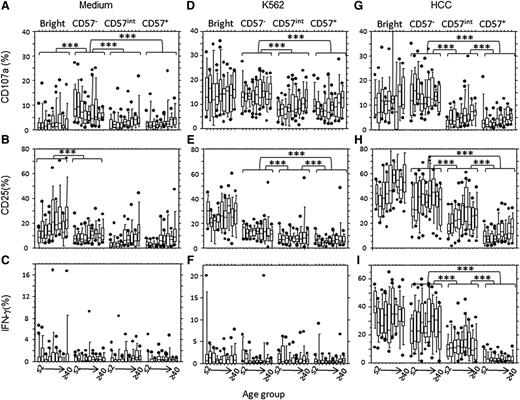
Age-associated changes in NK cell function. Example flow cytometry plots are shown for CD3− lymphocytes from a 1-year-old (A) and a 22-year-old (B), cultured in medium alone (top) or stimulated with high concentrations of IL-12+ IL-18 (HCC, bottom) and assayed for degranulation (CD107a), CD25, and IFN-γ expression. (C-K) NK cells were assayed for degranulation (C-E), CD25 (F-H), or IFN-γ (I-K) expression after in vitro culture in medium alone (C,F,I) or with K562 target cells (D,G,J) or IL-12 + IL-18 (HCC; E,H,K). Horizontal bars represent median values, boxes extend from the 25th to the 75th percentile, and whiskers represent the 95th percentiles. Asterisks denote significant age-related trends for frequencies of NK cells expressing CD107a, CD25, or IFN-γ (**P < .01;*** P< .001, F-test).
Age-associated changes in NK cell function. Example flow cytometry plots are shown for CD3− lymphocytes from a 1-year-old (A) and a 22-year-old (B), cultured in medium alone (top) or stimulated with high concentrations of IL-12+ IL-18 (HCC, bottom) and assayed for degranulation (CD107a), CD25, and IFN-γ expression. (C-K) NK cells were assayed for degranulation (C-E), CD25 (F-H), or IFN-γ (I-K) expression after in vitro culture in medium alone (C,F,I) or with K562 target cells (D,G,J) or IL-12 + IL-18 (HCC; E,H,K). Horizontal bars represent median values, boxes extend from the 25th to the 75th percentile, and whiskers represent the 95th percentiles. Asterisks denote significant age-related trends for frequencies of NK cells expressing CD107a, CD25, or IFN-γ (**P < .01;*** P< .001, F-test).
Spontaneous NK cell degranulation could be attributed to CD56dimCD57− cells (Figure 5A), whereas spontaneous expression of CD25 and IFN-γ production were restricted to the CD56bright subset (Figure 5B-C). CD107a and CD25 expression were observed in all NK cell subsets after incubation with K562 cells. Although this did not vary with age, it was higher in CD57− cells than in CD57int and CD57+ cells (Figure 5D-E), consistent with patterns of expression of the NKp30 activating receptor (which binds B7-H6 on K562 cells).31,32 As expected, K562 cells induced little IFN-γ secretion from any NK cell subset (Figure 5F).
NK cell function reflects CD57 expression, irrespective of age. Bright (CD56brightCD57−), CD57− (CD56dimCD57−),CD57int (CD56dimCD57int) and CD57+ (CD56dimCD57+) NK cell subsets were analyzed for CD107a (A,D,G), CD25 (B,E,H), or IFN-γ (C,F,I) after in vitro culture in medium alone (A-C), with K562 target cells (D-F) or with IL-12 + IL-18 (HCC; G-I). Horizontal bars represent median values, boxes extend from the 25th to the 75th percentile, and whiskers represent the 95th percentiles. There were no significant age-related trends in response within any of the subsets. Asterisks denote statistically significant differences between CD57−, CD57int, and CD57+subsets (P < .001 for all comparisons, Wilcoxon-signed rank).
NK cell function reflects CD57 expression, irrespective of age. Bright (CD56brightCD57−), CD57− (CD56dimCD57−),CD57int (CD56dimCD57int) and CD57+ (CD56dimCD57+) NK cell subsets were analyzed for CD107a (A,D,G), CD25 (B,E,H), or IFN-γ (C,F,I) after in vitro culture in medium alone (A-C), with K562 target cells (D-F) or with IL-12 + IL-18 (HCC; G-I). Horizontal bars represent median values, boxes extend from the 25th to the 75th percentile, and whiskers represent the 95th percentiles. There were no significant age-related trends in response within any of the subsets. Asterisks denote statistically significant differences between CD57−, CD57int, and CD57+subsets (P < .001 for all comparisons, Wilcoxon-signed rank).
CD57− NK cells (but not CD57int or CD57+ cells) degranulated extensively in response to cytokine stimulation (Figure 5G), and cytokine-induced CD25 expression and IFN-γ production declined with progressive NK cell differentiation, being highest in the CD56bright subset and lowest in the CD56dim CD57+ subset (Figure 5H-I). Although there was a trend for increasing CD107a and CD25 expression with increasing age in CD57int and CD57+ NK cells after cytokine stimulation (Figure 5G-H), this was only significant when comparing the very youngest and very oldest age groups (P < .01, analysis of variance).
Thus, although subtle age-associated changes in NK cell function may be evident within subsets, changing NK cell function with age is primarily a result of the changing proportion of cells within subsets.
Effect of NKG2C genotype on NK cell numbers and phenotype
Lack of NKG2C expression because of deletion of the NKG2C locus has been reported in several populations.27,33-35 Nineteen of 181 individuals tested here (10.5%) were NKG2C−/− (and lacked surface expression of NKG2C), whereas 68 individuals (37.6%) were heterozygotes, giving a haplotype frequency of 29.3%. NKG2C−/− individuals were distributed evenly across age groups and between the sexes (Table 1).
NKG2C genotype did not affect frequencies of total, CD56bright, or CD56dim NK cells, although, consistent with published data,34 NKG2C−/− children younger than 10 years had lower absolute numbers of NK cells when compared with NKG2C+/− children (supplemental Figure 7). However, NKG2C−/− individuals had significantly lower frequencies of CD56dim CD94+ NK cells than did NKG2C+/− and NKG2C+/+ individuals (Figure 6A). Absolute numbers of NKG2A+ cells were unaffected by genotype (supplemental Figure 8B), whereas absolute numbers of CD94+ cells were significantly lower among NKG2C−/− individuals (supplemental Figure 8A). This is consistent with CD94+NKG2A+ to CD94+NKG2C+ ratios being determined by expansion within the CD94+NKG2C+ subset, rather than by conversion of NKG2A+ cells to NKG2C+ cells. A significant gene dosage effect was observed, with NKG2C+/− individuals having intermediate frequencies (Figure 6B) and numbers (supplemental Figure 8) of CD94+NKG2A+ and CD94+NKG2C+ cells. A modest decrease in the MFI for NKG2C expression was observed in NKG2C+/− compared with NKG2C+/+ individuals, although this did not reach statistical significance (supplemental Figure 9). NKG2C−/− children (younger than 10 years) had significantly lower frequencies of CD57+ NK cells than did heterozygous and homozygous NKG2C+ children, with a reciprocal increase in both CD57− and CD57int NK cells (Figure 6C). This effect was absent in individuals older than 10 years.
Effect of NKG2C genotype on NK cell maturation phenotype and HCMV antibody titer. (A) Frequency of CD94+ cells within the CD56dim NK cell population in individuals with zero (NKG2C−/−), 1 (NKG2C+/−), or 2 (NKG2C+/+) copies of the NKG2C gene. (B) Effect of NKG2C genotype on the frequencies of CD94+ NK cells expressing either NKG2A+(NKG2C−) or NKG2C+(NKG2A−) cells. (C) Effect of NKG2C genotype on the frequency of CD57−, CD57int, and CD57+ NK cells in subjects younger than 10 and 10 or more years of age. (D) Anti-HCMV antibody titers by age (years) and NKG2C−genotype. Horizontal bars represent median values, boxes extend from the 25th to the 75th percentile, and whiskers represent the 95th percentiles. Asterisks denote statistically significant differences between genotypes for all comparisons shown (*P < .05, ***P < .001, analysis of variance).
Effect of NKG2C genotype on NK cell maturation phenotype and HCMV antibody titer. (A) Frequency of CD94+ cells within the CD56dim NK cell population in individuals with zero (NKG2C−/−), 1 (NKG2C+/−), or 2 (NKG2C+/+) copies of the NKG2C gene. (B) Effect of NKG2C genotype on the frequencies of CD94+ NK cells expressing either NKG2A+(NKG2C−) or NKG2C+(NKG2A−) cells. (C) Effect of NKG2C genotype on the frequency of CD57−, CD57int, and CD57+ NK cells in subjects younger than 10 and 10 or more years of age. (D) Anti-HCMV antibody titers by age (years) and NKG2C−genotype. Horizontal bars represent median values, boxes extend from the 25th to the 75th percentile, and whiskers represent the 95th percentiles. Asterisks denote statistically significant differences between genotypes for all comparisons shown (*P < .05, ***P < .001, analysis of variance).
Finally, to explore whether the NKG2C genotype might affect control of HCMV, we examined the relationships among age, genotype, and anti-HCMV antibody titer (Figure 6D). Anti-HCMV antibody titers were markedly and significantly higher in NKG2C−/− than in NKG2C+/+ children (younger than 10 years) (Figure 6D), suggesting that inferior control of HCMV infections in these children may lead to more frequent reactivation and boosting of antibody responses. This effect was not observed in older individuals and appeared to be specific for HCMV, as no effect of NKG2C genotype was observed on titers of antibodies to childhood vaccine antigens or EBV (supplemental Figure 10). One explanation for this is that lack of NKG2C+ NK cells may hinder control of HCMV, such that the ability to control HCMV viral load (as reflected by anti-HCMV titer) develops more slowly in children who lack NKG2C.
Discussion
It is increasingly appreciated that NK cells are genetically, phenotypically, and functionally diverse, both at the human population level36 and within individuals.37 Moreover, NK cells differentiate through the life course, reflecting the interplay of genes and environment. These adaptations substantially modify NK cell function20,38,39 and are beginning to be associated with health outcomes.9 Age is a major determinant of NK cell phenotype and function,18-22 but it is not yet clear whether this is a result of primary, age-intrinsic processes or whether age is simply a marker for cumulative environmental exposures. HCMV infection is a major confounder of the association between age and NK cell function,11,26,40 but HCMV status is not reported in many published studies, hindering data interpretation. To unpick these issues, detailed phenotypic and functional studies are required across the entire age span and among genetically diverse populations in different environments. The data presented here represent the most comprehensive study to date of NK cell phenotype and function from infancy to mature adulthood and the first such study in an African community and in a population where confounding by HCMV infection status is minimized because of near-universal HCMV infection in infancy.
We previously identified an apparently transitional population of CD56dim NK cells with intermediate CD57 expression (CD57int), expressing intermediate levels of CD16, CD62L, IL-12R, and IL-18R and with a capacity for degranulation, CD25 expression, and IFN-γ production between CD57− and CD57+ NK cells.6 Here we observe that although frequencies of CD57− cells decrease and frequencies of CD57+ cells increase with age, a small but persistent population of CD57int NK cells is present at all ages, suggesting that differentiation of CD57− to CD57+ NK cells occurs at a similar rate throughout the life course. If so, age-related changes in CD57− and CD57+ NK cell frequencies must reflect differential rates of loss or proliferation of these 2 subsets, rather than changing rates of cell conversion. Rates of both apoptosis and proliferation are reportedly very high in human NK cells,41 but whether these rates differ between CD57− and CD57+ NK cells is unknown.
One striking observation in this population is the very high frequency of fully differentiated CD56dimNKG2C+CD57+ NK cells in very young children; these cells represent up to 50% of all NK cells in 1- to 2-year-olds and up 80% of cells in 6- to 9-year-olds, with the mature adult range (∼30% to 70%) being reached by the age of 10 years. In Europeans, proportions of CD57+ NK cells range from zero at birth (cord blood) to median values of ∼50% in adults,20 with values being higher in HCMV+ individuals (30%-70%) than in HCMV− subjects (25%-50%).42 Although we could not compare our data with a fully age-matched, low-HCMV prevalence cohort, the frequency of CD57+ NK cells in HCMV-seropositive adult Gambians is significantly higher than in an age-matched HCMV-seropositive UK cohort, confirming more rapid or extensive NK cell differentiation among Gambians (supplemental Figure 11). This may reflect either HCMV infection much earlier in life in The Gambia or a higher prevalence of other infections that further expand the NKG2C+CD57+ NK cell population in HCMV+ individuals.43 Data on HCMV+ and HCMV− European children are needed to confirm this.
Interestingly, HCMV and EBV coinfection did not affect NK cell phenotype or function. EBV coinfection has been associated with more extensive NK cell differentiation compared with HCMV alone in some European studies,7 but not in a recent US study,30 suggesting that perinatal HCMV infection alone is sufficient to drive NK cell differentiation or that infections other than EBV may also have an effect in this Gambian cohort. Of note, the biphasic kinetic of NK cell differentiation is not accompanied by a similar biphasic differentiation of T-cell populations, which is consistent with the suggestion that HCMV infection independently affects T-cell and NK cell populations.44
Age-related differences in NK cell function were entirely a result of differences in the proportions of CD57− and CD57+ NK cells. In Caucasian adults, cytokine-induced degranulation, CD25 expression, and IFN-γ production all decline with increasing levels of CD57 expression,2,4-6 in parallel with reduced expression of IL-12 and IL-18 receptors,6 whereas CD57 expression has much less effect on responses receptor crosslinking.6 This association between CD57 expression and NK cell function also holds true in The Gambia and in children as young as 3 to 5 years of age. Because CD57 expression is, to a large extent, driven by HCMV, it appears that infection with HCMV very early in life rapidly skews the entire NK cell population to missing/altered-self/antibody-dependent cytotoxicity at the expense of cytokine-driven responses.6,45 This skewing of NK cell function is much more marked among Gambian adults than among age-matched HCMV seropositive UK adults, again presumably reflecting an earlier age of HCMV infection or increased prevalence of coinfections in The Gambia (supplemental Figure 11). Altered NK cell function so early in life could contribute to associations among perinatal HCMV infection, slower growth, and increased rates of hospitalization, as observed in Zambian children.46
In line with the near-universal HCMV infection in infancy in our cohort and the well-documented expansion of CD57+NKG2C+ NK cells in HCMV+ individuals,11 frequencies of NKG2C+ cells were high in all age groups. Frequencies of NKG2C+ NK cells were lower in very young children than in older age groups, but adult frequencies were achieved by the age of 6 to 9 years, suggesting that expansion of the NKG2C+ subset begins very quickly after HCMV infection and may continue for some years. This is consistent with data from transplant recipients with acute HCMV infection or HCMV reactivation, where frequencies of NKG2C+ NK cells increase within a month of infection/reactivation and continue to increase for at least 12 months,14,26,40 and with reports of significantly higher frequencies of NKG2C+ NK cells in HCMV+ compared with HCMV− children younger than 2 years.47
In Caucasian adults, the NKG2C+ NK cells induced by HCMV infection tend to coexpress CD57.14 This was also the case here, although both the frequency of NKG2C+ cells expressing CD57 and the median MFI of CD57 expression were lower in children younger than 2 years than in older individuals. In contrast, CD57 is expressed only at low intensity on NKG2A+ NK cells at all ages. These observations are consistent with a model in which peptides from HCMV UL40 bind to HLA-E, stabilizing it at the surface of infected cells, where it drives activation, proliferation, and differentiation (including expression of CD57) of NK cells expressing NKG2C (the activating receptor on NK cells for HLA-E) while simultaneously inhibiting proliferation and differentiation of cells expressing NKG2A (the inhibitory HLA-E receptor).10,48-50
Although CD94/NKG2C and CD94/NKG2A are not the only NK cell receptors for HCMV,51-53 lack of NKG2C was clearly linked to delayed NK cell differentiation and maturation. NKG2C+/− heterozygotes had lower frequencies of NKG2C+ NK cells than did NKG2C+/+ individuals (consistent with a previous report33 ), and the frequency and absolute number of CD94+ cells was positively associated with NKG2C copy number, consistent with the hypothesis that NKG2C+ NK cell numbers expand by proliferation, rather than by transformation from NKG2A+ cells. Importantly, however, a high proportion of CD56dim NK cells in NKG2C−/− individuals lack expression of CD94/ NKG2A, as well as CD94/NKG2C, raising questions about which other receptors might be expressed on these cells to maintain NK cell homeostasis and HCMV latency. HCMV reactivation in recipients of NKG2C−/− stem cells drives differentiation of functional KIR+NKG2A- NK cells,53 suggesting that activating KIR may compensate for lack of CD94/NKG2C. Stable expansions of KIR+ NKG2A−NKG2C− NK cells have also been observed in HCMV-seropositive adults.54
Consistent with activation and expansion of NKG2C+ cells before their acquisition of CD57, proportions of CD57+ NK cells were significantly lower in NKG2C−/− subjects than in those with 1 or more copies of NKG2C, and in particular for children younger than 10 years. The magnitude of this effect is remarkable, achieving statistical significance despite the rather small number of NKG2C−/− subjects, and is likely to be highly biologically relevant. It would be interesting to know whether delayed NK cell differentiation in HCMV-infected NKG2C−/− subjects is seen in other populations and whether it confers any survival advantage or whether this is offset by impaired control of HCMV (as implied by the significantly higher anti-HCMV antibody titers). These studies will need to be large enough to achieve statistical power, which will depend on both the prevalence of the NKG2C-null haplotype and of HCMV. The 29.3% haplotype frequency of the NKG2C deletion in our African cohort is higher than that recorded elsewhere.33,35,55 Whether the frequency of this haplotype is linked to current or historic intensities of HCMV infection might also merit further investigation.
Despite our study cohort being almost uniformly HCMV-seropositive, considerable heterogeneity is observed in NK cell phenotype and function within each age group. Although some of this is heterogeneity may be genetically determined and/or stochastic,37 exposure to infections in addition to HCMV may also affect NK cell maturation.7,15,17,56 Further studies are needed to determine whether this is simply a cytokine-driven expansion, and thus likely to occur in response to many acute inflammatory stimuli, or whether some pathogens express specific ligands for CD57+NKG2C+ NK cells.
In summary, our study has revealed rapid phenotypic and functional differentiation of peripheral NK cells in a population with extremely high rates of perinatal HCMV infection. Intriguingly, NK cell phenotype seems to be highly dependent on the expression of NKG2C, reaffirming the notion that signaling via NKG2C is causally linked to NK cell differentiation. Further studies are now warranted to evaluate the effect of early HCMV infection on the ability of NK cells to contribute to protection from other infections throughout the life course.
The online version of this article contains a data supplement.
There is an Inside Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We would like to thank Professors Andy Hall and Andrew Prentice for facilitating the initiation of this project; Kerry Jones for logistical and technical support; Medical Research Council fieldworkers Sulayman Bah, Wally Kamara, and Lamin Jatta for recruiting subjects; Bai Lamin Dondeh for database management; the staff of the Medical Research Council International Nutrition Group and Medical Research Council Gambia (Keneba) for their support; and the villagers of Keneba, Manduar, and Kantong Kunda for participating in the study. We also thank Professor Beate Kampmann and colleagues (Medical Research Council Gambia, Fajara) for advice and access to laboratory facilities. This study was funded by the UK Medical Research Council (G1000808).
Authorship
Contribution: M.R.G. designed research, performed experiments, analyzed and interpreted data, and wrote the manuscript; M.J.W. designed and performed research and analyzed and interpreted data; A.D. designed and performed research; C.M.N. performed experiments and analyzed data; A.G. performed research; C.B. analyzed data; S.E.M. designed research and coordinated recruitment of study subjects; and E.M.R. designed research, supervised data collection, directed data analysis, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Eleanor M. Riley, London School of Hygiene and Tropical Medicine, Keppel St, London, WC1E 7HT, United Kingdom; e-mail: eleanor.riley@lshtm.ac.uk.