In this issue of Blood, El Hajj et al report that the synthetic retinoid ST1926 downregulates the oncoprotein Tax and induces apoptosis and growth arrest of adult T-cell leukemia (ATL) cells.1
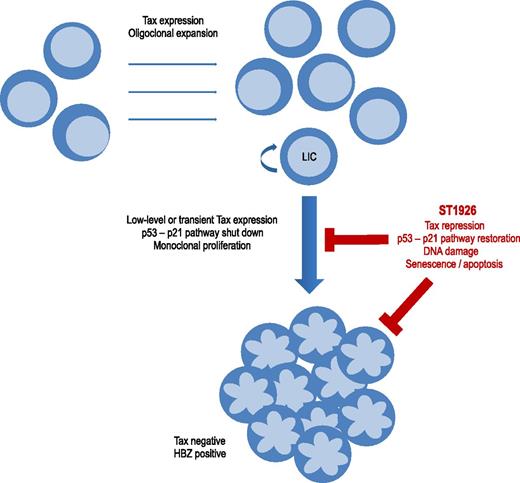
Schematic representation of ST1926 repression of Tax and apoptosis of ATL cells. Tax expression induces oligoclonal expansion of HTLV-1–infected CD4 T cells and deregulates signaling pathways including p53-p21 or DNA repair. ATL monoclonal proliferation occurs after decades of evolution in 2% to 7% of HTLV-1–infected patients. Cells having shut down Tax expression escape anti-Tax cytotoxic T lymphocytes and are preferentially selected. ATL cells have a Tax-negative and HBZ-positive phenotype. Transient or low-level Tax expression occurs, particularly in leukemia-initiating cells. ST1926 downregulates Tax expression and reactivates the p53-p21 signaling pathway. ST1926 induces massive and p53-independent apoptosis of leukemic cells.
Schematic representation of ST1926 repression of Tax and apoptosis of ATL cells. Tax expression induces oligoclonal expansion of HTLV-1–infected CD4 T cells and deregulates signaling pathways including p53-p21 or DNA repair. ATL monoclonal proliferation occurs after decades of evolution in 2% to 7% of HTLV-1–infected patients. Cells having shut down Tax expression escape anti-Tax cytotoxic T lymphocytes and are preferentially selected. ATL cells have a Tax-negative and HBZ-positive phenotype. Transient or low-level Tax expression occurs, particularly in leukemia-initiating cells. ST1926 downregulates Tax expression and reactivates the p53-p21 signaling pathway. ST1926 induces massive and p53-independent apoptosis of leukemic cells.
ATL is an aggressive lymphoid proliferation caused by human T-cell leukemia virus type 1 (HTLV-1), which is also the etiologic agent of HTLV-1–associated myelopathy/tropical spastic paraparesis. The estimated lifetime risk of developing ATL in HTLV-1 carriers is 2% to 7%, and the disease usually occurs ≥20 to 30 years after HTLV-1 infection. ATL is classified as a peripheral T-lymphocytic malignancy of CD4+ T phenotype. The diversity in clinical features and evolution has led to its classification into 4 clinical subtypes: smoldering, chronic, acute, and lymphoma-type ATL. Patients with acute or lymphoma forms have high-risk ATL (HR-ATL), and their poor prognosis is due to rapid proliferation, marked immunosuppression, and resistance to chemotherapy. Although the combination of zidovudine and interferon-α (IFN-α) improves response rate and survival,2 almost all HR-ATL patients relapse. New therapeutics are therefore highly needed.
Retinoid acids are regulators of cellular proliferation and differentiation. Natural retinoids such as all-trans retinoic acid (ATRA) are currently used as therapeutic agents in human cancers, mainly acute promyelocytic leukemia.3 Resistance to ATRA is frequent in ATL cells, and a pilot study showed only a partial response in less than half of ATL patients.4 Synthetic retinoids, such as N-(4-hydroxyphenyl)retinamide (HPR), 6-[3-(1-adamantyl)-4-hydroxyphenyl]-2-naphthalene carboxylic acid (CD437), and (2E)-3-[3′-(1-adamantyl)-4′-hydroxy[1,1′-biphenyl]-4-yl]-2-propenoic acid (ST1926), have been developed to overcome resistance and attenuate side effects. These atypical retinoids are able to induce apoptosis in malignant cells through both retinoic acid receptor–dependent and –independent mechanisms. HPR has been shown to inhibit growth and induce apoptosis in HTLV-1–transformed cells.5
El Hajj et al evaluated the preclinical efficacy of ST1926 in ATL.1 At pharmacologically relevant concentrations, ST1926 causes G1 cell cycle arrest and massive apoptosis in HTLV-1–infected cell lines. A growth inhibition is observed with ATL primary cells but not peripheral blood mononuclear cells from healthy donors. ST1926-induced cell death is partially caspase dependent. The study provides further evidence that ST1926 is a DNA-damaging agent. ST1926 upregulates p53, but the growth suppressive effect is p53 independent. The authors also show that oral administration of ST1926 reduces leukemic burden and prolongs survival in a murine ATL model developed by Hasegawa et al.6 The effect of ST1926 on ATL cells was more pronounced than those of HPR or CD437. Interestingly, among these synthetic retinoids, only ST1926 downregulates the levels of the viral oncoprotein Tax in HTLV-1–infected cell lines. ST1926-induced downregulation of Tax was confirmed by a reduction of Tax mRNA levels in spleen leukemic cells and Tax DNA in treated mice with ATL.
Tax transactivates viral expression but also deregulates apoptosis, cell cycle, and DNA repair. Tax supports the oligoclonal expansion of HTLV-1–infected T lymphocytes and plays a key role in the initiation of the multistep process of leukemogenesis (see figure). Paradoxically, Tax protein is usually not detectable in HTLV-1–infected peripheral blood mononuclear cells and ATL cells, and tax expression in vivo can only be assessed at the transcript level. In vivo dynamic studies support the persistence of continuous low-level or transient expression of Tax protein. The current view is that, Tax being the main target of the host’s cytotoxic T lymphocytes, cells that have silenced Tax expression are preferentially selected during disease progression. Several mechanisms of Tax silencing have been described, including provirus deletion in the 5′ long terminal repeat, mutations in the tax gene, epigenetic modulation, and silencing by other regulatory viral proteins such as p30 or HTLV-1 bZIP factor (HBZ).
A question that has not been addressed is the effect of retinoids on the expression of HBZ, the other HTLV-1 oncogene. HBZ is consistently expressed in ATL primary cells, and evidence is accumulating about its critical role in the maintenance of HTLV-1–induced transformation.7 Leukemic cells from the Tax transgenic murine model do not express HBZ, and the effect on Tax may not be sufficiently relevant in patients with HR-ATL.
The mechanism by which decreased Tax protein level is mediated by ST1926 action is also not yet elucidated. In a previous study, El Hajj et al reported that As2O3 and IFN-α trigger Tax proteolysis and target leukemia-initiating cells.8 They suggest that ST1926 may downregulate Tax through proteasome degradation, with subsequent restoration of the Tax-inhibited p53-p21 pathways and apoptosis or senescence in quiescent leukemic cells.1 They conclude that the association of ST1926 with As2O3 and IFN-α may abrogate ATL-initiating activity and favor long-term remissions.
The infected leukemic stem cell hypothesis has emerged recently as a potent mechanism for relapse in patients who have reached remission. Although the existence of chemotherapy-resistant cancer stem cells is supported by the Tax transgenic mouse ATL model,9 their characteristics cannot be transposed to leukemic ATL-CD4+ mature cells. Allogeneic hematopoietic stem cell transplantation may be the only long-term remission and potentially curative strategy.10 Further studies on potential drugs, such as ST1926, should investigate the effect on leukemic stem cells and their use in the context of allogeneic transplant and maintenance therapy.
Conflict-of-interest disclosure: The authors declare no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal