In this issue of Blood, Khoder et al show that human IgM memory B cells possess immunoregulatory properties including IL-10 production, analogous to transitional B cells, and that after allogeneic stem cell transplantation, IL-10–producing B cells are deficient in patients with chronic graft-versus-host disease (cGVHD).1
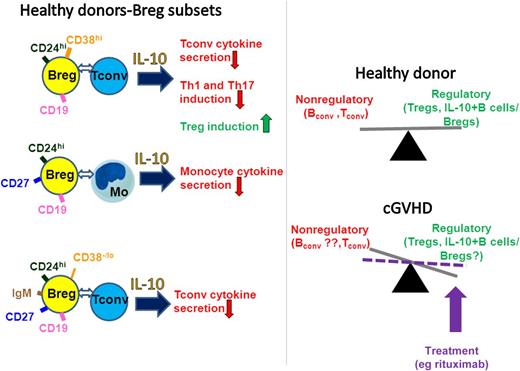
Breg subsets from healthy individuals are present within the CD19+CD24hiCD38hi, CD19+CD24hiCD27+, and IgM memory B-cell (current study) populations and inhibit immune responses in part through IL-10 and direct interactions with the target cells. In healthy individuals, there is a balance between regulatory (including Tregs and Bregs/IL-10–expressing B cells) and the nonregulatory immune compartments such as conventional (conv) B and T cells. The study by Khoder et al suggests that the balance between IL-10+ B cells and the nonregulatory cells is disturbed in severe cGVHD, but that it may be reset by treatments such as rituximab. Tconv, conventional T cells; Mo, monocytes.
Breg subsets from healthy individuals are present within the CD19+CD24hiCD38hi, CD19+CD24hiCD27+, and IgM memory B-cell (current study) populations and inhibit immune responses in part through IL-10 and direct interactions with the target cells. In healthy individuals, there is a balance between regulatory (including Tregs and Bregs/IL-10–expressing B cells) and the nonregulatory immune compartments such as conventional (conv) B and T cells. The study by Khoder et al suggests that the balance between IL-10+ B cells and the nonregulatory cells is disturbed in severe cGVHD, but that it may be reset by treatments such as rituximab. Tconv, conventional T cells; Mo, monocytes.
The term regulatory B cells (Bregs), analogous to regulatory T cells (Tregs), was first coined by Mizoguchi et al in 2002 to describe mouse B cells with immune inhibitory function.2 This contrasted with the classic B-cell functions such as antibody production or antigen presentation associated with driving immune responses. Although the existence of suppressor B cells had been known since the 1970s, interest in the characterization of Breg compartment has recently gathered momentum in part because of their potential to induce tolerance similar to Tregs. Moreover, with increasing use of B cell–targeted therapies such as rituximab, it is becoming clear that strategies are needed to spare (or to delete in case of malignancies) Bregs from B-cell depletion regimens to avoid potential development of immune pathology and to fine-tune the treatments such that Bregs are selectively and preferentially expanded (or deleted) in the reconstituted B-cell population after B-cell depletion. Despite efforts to identify the precise phenotype of Bregs, cell-surface markers or transcription factors specific to Bregs have remained elusive, hampering studies to isolate and define them. Instead, Breg characterization has relied exclusively on assessing their suppressive activity in vivo or by in vitro assays in part through the use of sort-purified B-cell populations. Several Breg subsets have been described in mice and humans and they all share the ability or at least the competency to express the antiinflammatory cytokine IL-10, despite the caveat that IL-10 also promotes B-cell maturation into plasma cells.3 Blair et al were the first to describe Bregs in humans, identifying them in peripheral blood within the CD19+CD24hiCD38hi subset, a phenotype previously associated with immature transitional B cells.4 Compared with CD19+CD24hiCD38–/lo memory or CD19+CD24intCD38int mature B-cell subsets, CD19+CD24hiCD38hi cells produced more IL-10 after CD40 stimulation, although their ability to express other cytokines, including pro- or antiinflammatory types, was not assessed. These cells suppressed the production of proinflammatory cytokines by CD4+ T cells, in part through the production of IL-10 and via cell-to-cell contact with target CD4+ T cells requiring CD80-CD86 cell-cell interactions. This subset can inhibit T helper 1 (Th1) and Th17 differentiation while favoring Treg induction from naïve CD4+ T cells, partly through the provision of IL-10.5 In support of their regulatory properties, patients with autoimmune diseases have functional and/or numerical deficiency of this subset,4,5 and responders to rituximab treatment show an increased ratio of CD19+CD24hiCD38hi B cells to memory B cells.6 Iwata et have described another human Breg within CD19+CD24hiCD27+ cells, sharing markers with activated and memory (CD27) B cells.7 These cells, referred to as B10 cells, have been extensively characterized as IL-10–competent CD5+CD1dhi Bregs in mice. Interestingly, CD19+CD24hiCD27+ cells stimulated through CD40 and toll-like receptors reduced tumor necrosis factor-α production by monocytes partly through the production of IL-10. However, their ability to inhibit cytokine production by CD4+ T cells was equal to the reciprocal CD19+CD24loCD27– population and was IL-10–independent.
The present study by Katona et al reconciles some of the different phenotypes of human Bregs (see figure) by defining an IgM+ memory Breg subset that is suppressive against T cells within the CD27+CD24hi population described by Iwata et al.7 The identification of this novel IgM+ Breg subset also explains why the parental CD19+CD24hiCD38–/lo memory population described by Blair et al had expressed suboptimal IL-10 levels.4 IgM memory and transitional Breg subsets expressed IL-10 at comparable levels, and both subsets inhibited CD4+ proinflammatory cytokine production partly through IL-10 production and CD80/CD86 interactions. Interestingly, transitional and IgM memory Bregs possessed the same suppressive capacity as Tregs on a per-cell basis in inhibiting T-cell activation.
The authors then went on to examine Bregs in a cohort of patients with cGVHD, known to have altered B-cell homeostasis, impaired T-cell functions, and deficiency of Tregs. Peripheral B-cell phenotypic analysis showed that their cGVHD patient cohort had lower numbers of circulating transitional B cells but comparable numbers of peripheral IgM memory B-cell subsets compared with patients without cGVHD or healthy individuals. But the key finding of their study was that the cGVHD patient had a lower frequency and lower absolute numbers of IL-10–producing B cells in response to CD40 stimulation with significantly higher Th1/IL-10+ B-cell ratios (see figure). These data suggest that patients with cGVHD may have impaired Breg activity, although evaluation of the suppressive activity of Breg subsets on T cells using purified IgM memory and transitional B cells from cGVHD patients was not done. Despite the small cohort of patients with cGVHD that were studied (n = 11), those with severe or moderate cGVHD had lower IL-10+ B cells compared with those with mild cGVHD, raising the possibility that IL-10+ B-cell deficiency contributes to the previously described persistent B-cell activation state, and autoimmune phenomenon described in treatment-resistant cGVHD patients.8-10 Although the current study convincingly demonstrates that Breg subsets are enriched in transitional and IgM+ memory (CD27+) subsets, others have shown that some of the potentially pathogenic circulating B-cell subsets in treatment-resistant cGVHD have markers of naïve and transitional B-cell phenotype8,9 and that CD27+ B cells in patients with cGVHD are activated, constitutively producing IgG ex vivo.10 Clearly, the current study offers new challenges, including the need to precisely define and characterize the phenotype of regulatory vs activated B-cell subsets in cGVHD and to establish the lineage relationship among the subsets. Such studies will help not only with our understanding of the role of the Breg subsets relative to other B cell populations in the pathophysiology of cGVHD, but also lead to the identification of markers of response to cGVHD treatment options including B cell–targeted therapies.
Conflict of interest disclosure: The author declares no competing financial interests.