In this issue of Blood, Newson et al determine the dynamics of inflammatory cell recruitment and their diversification into functionally distinct, cellular subsets in a previously uncharacterized “postresolution” phase of inflammation.1 The postresolution tissue retains some of the recruited populations of monocytic and lymphoid cells and remains in a state of “adaptive homeostatsis” long after inflammation has resolved.
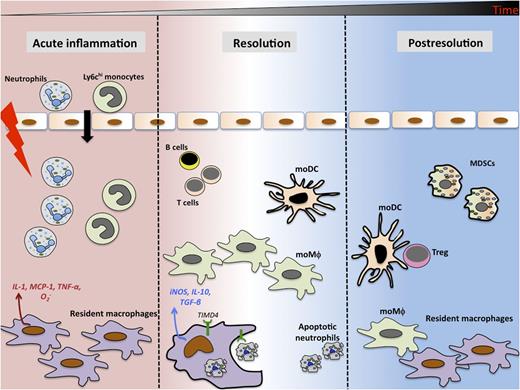
The acute phase of inflammatory response is accompanied by rapid (within minutes) influx of neutrophils and monocytes in response to soluble mediators released by tissue resident cells. During resolution, resident macrophages clear apoptotic neutrophils and make iNOS and proresolving cytokines such as interleukin-10 (IL-10) and transforming growth factor-β (TGF-β). Monocyte-derived macrophages (moMφ) and MoDCs persist in the tissue along with MDSCs and lymphocytes long after classical resolution. MoDCs preferentially drive T-cell proliferation and generation of adaptive immunity in the postresolution phase.

The acute phase of inflammatory response is accompanied by rapid (within minutes) influx of neutrophils and monocytes in response to soluble mediators released by tissue resident cells. During resolution, resident macrophages clear apoptotic neutrophils and make iNOS and proresolving cytokines such as interleukin-10 (IL-10) and transforming growth factor-β (TGF-β). Monocyte-derived macrophages (moMφ) and MoDCs persist in the tissue along with MDSCs and lymphocytes long after classical resolution. MoDCs preferentially drive T-cell proliferation and generation of adaptive immunity in the postresolution phase.
The host inflammatory response is a complex, well-orchestrated process temporally regulated by the release of various cytokines, chemokines, lipid mediators, and immune cellular subsets. In the acute phase of inflammatory response, following tissue injury, resident macrophages and mast cells release chemical mediators (eicosanoids, chemokines, and proinflammatory cytokines) that initiate rapid influx of neutrophils and monocytes into the tissue. After neutralization of the inciting stimuli, neutrophils undergo apoptosis and are cleared by efferocytosing macrophages, subsequently resulting in resolution and restoration of normal tissue architecture.2 Several studies have elegantly demonstrated that resolving inflammation is a complex process comprising multiple regulatory events such as phenotypic transformation of macrophages from inflammatory to antiinflammatory type, catabolism of proinflammatory cytokines, and release of proresolving lipid mediators that synergistically promote wound healing.3-5 Persistent, unresolving inflammation is often correlated with severity and unfavorable outcomes in various inflammatory diseases such as arthritis, lupus, and periodontitis. Insight into the cellular players and molecular signals that drive resolution is critical in devising therapeutic interventions for the treatment of chronic inflammatory diseases.
Several functionally specialized immune cell types shut down inflammation. Macrophages are a highly plastic, functionally diverse, myeloid cell population that produces a battery of immune-modulating mediators to regulate both pro- and antiinflammatory arms of immune response. They can undergo phenotypic alterations and reprogram their cell intrinsic effector functions to alter the tissue microenvironment.6 Myeloid-derived suppressor cells (MDSCs) are another subset of highly heterogeneous immune regulatory cells that limit T-cell proliferation, produce immunosuppressive inducible nitric oxide synthase (iNOS), and participate in a range of pro- and antiinflammatory pathways.7 However, little is known about the dynamics of how these cellular subsets are recruited to the inflammatory site, roles they play in altered tissue microenvironment, and whether their dysregulation perpetuates inflammation.
Newson et al1 demonstrate a previously uncharacterized “postresolution” phase of inflammation that persists weeks after classical resolution and is dominated by the presence of resident macrophages, MDSCs, monocyte-derived macrophages, and monocyte-derived dendritic cells (moDCs). They present evidence that each of these monocytic subsets (identified based on lineage and activation makers) play distinct yet overlapping roles in creating a microenvironment conducive for the development of adaptive immune responses. In a model of low-dose zymosan-induced peritoneal injury, there is a rapid influx of neutrophils during the acute phase of inflammatory response (see figure). The neutrophils subsequently become apoptotic. Resident macrophages preferentially efferocytose apoptotic neutrophils and acquire an immunosuppressive phenotype by producing antiinflammatory cytokines TGF-β and interleukin-10. Resident macrophages from the peritoneal cavities of mice challenged with bacteria also exhibit similar properties and behavior. Furthermore, iNOS expression in resident macrophages dampened T-cell proliferation in the peritoneal cavity and promoted lymph node contraction. Although postresolution macrophages induced FoxP3 expression in CD4-positive T cells, moDCs enabled their proliferation, thus enriching the peritoneal cavity with CD4+, CD25+, or FoxP3 regulatory T cells (Tregs). Tregs are immunosuppressive populations of T cells that modulate the intensity of other immune cells and regulate immunity by maintaining tolerance to self-antigens.8 Treg development was hampered in mice injected with higher doses of zymosan, resulting in hyperinflammation and significantly higher inflammatory cell infiltrate. The authors speculate that the blunted development of Tregs might contribute to maladaptive immune responses and may predispose to autoimmune/chronic inflammatory disorders.
To summarize, Newson et al elegantly demonstrate that inflammatory resolution is not just a mere contraction of inflammatory cells, but a complex, well-regulated process comprising multiple proresolution cellular and molecular pathways. The recruited cells persist in the tissue for months after resolution and dictate the magnitude of subsequent inflammatory challenge. Resolving inflammation thus generates a microenvironment conducive for “bridging” innate and adaptive arms of immunity. Whether the phenomenon of “adaptive homeostasis” is restricted to the peritoneal cavity or is a common mechanism of resolution remains to be tested in other tissues and animal models.
Conflict-of-interest disclosure: The author declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal