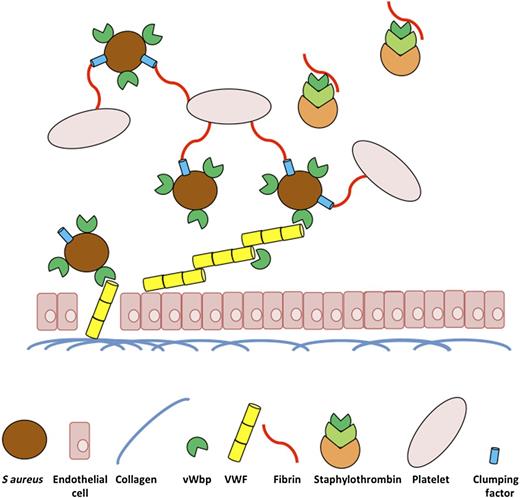
In this issue of Blood, Claes et al elegantly illustrate the importance of the Staphylococcus aureus von Willebrand factor binding protein (vWbp) in the initiation of vascular lesions under flow.1
A diagrammatic representation of the role of vWbp of S aureus in the initiation of vascular infections under physiological flow conditions. The underlying extracellular matrix of vascular tissue is exposed following damage to vascular endothelial cells. VWF released from these cells can then bind to collagen in the extracellular matrix and acts as a bridge to promote S aureus attachment to the damaged tissue via vWbp under shear stress. vWbp further enhances infected vascular lesions by promoting the attachment of platelets to the site of infection through its procoagulant role in staphylothrombin, converting soluble fibrinogen to fibrin and promoting the attachment of S aureus to platelets via clumping factor adhering to fibrin.
A diagrammatic representation of the role of vWbp of S aureus in the initiation of vascular infections under physiological flow conditions. The underlying extracellular matrix of vascular tissue is exposed following damage to vascular endothelial cells. VWF released from these cells can then bind to collagen in the extracellular matrix and acts as a bridge to promote S aureus attachment to the damaged tissue via vWbp under shear stress. vWbp further enhances infected vascular lesions by promoting the attachment of platelets to the site of infection through its procoagulant role in staphylothrombin, converting soluble fibrinogen to fibrin and promoting the attachment of S aureus to platelets via clumping factor adhering to fibrin.
There has been considerable discussion in the field with respect to the hierarchy of importance of different virulence factors of S aureus that contribute to the pathogenesis of endovascular infections such as infective endocarditis, an infection of the heart valve. Do the interactions of S aureus with serum proteins, platelets, or endothelial cells and exposed extracellular matrix proteins make the most significant contribution? This paper shows for the first time that vWbp is critical in establishing lesions on the surface of the in vivo vasculature under physiological flow via its von Willebrand factor (VWF) binding and procoagulant activity.
Previous research by Pappelbaum et al2 found that VWF is the host mediator of S aureus vascular endothelial cell binding. This study augments those findings by clearly identifying that the S aureus bacterial virulence factor, vWbp, is responsible for this interaction. The VWF binding protein of S aureus (vWbp) was first described in 2002.3 However, its role in endovascular infection by S aureus remained unclear until this study. Protein A, another S aureus surface protein, was originally identified as a VWF binding protein.4 However, under conditions of high shear stress, Protein A does not promote the adherence of S aureus to VWF.2 Claes et al show that a combination of its adhesive and procoagulant activity contributes to the ability of vWbp to promote endovascular infection, an effect that becomes apparent under physiologically relevant shear stress conditions.
This paper also shows that exogenously produced vWbp associates with S aureus cells to promote the observed VWF adherence under physiological shear stress. Therefore, although the Pappelbaum et al2 study indicated that teichoic acids are involved in the interaction of S aureus with VWF, it is tempting to speculate that the reduction in adherence to vascular endothelial cells under flow that was observed in a S aureus strain deficient in wall teichoic acid is, in part, is a result of a reduced association of vWbp with S aureus due to the lack of wall teichoic acids on its cell surface. Further studies may demonstrate whether this is, in fact, the case.
It will be interesting to follow-up on the observations in this study by studying the VWF binding capacity of other clinically relevant bloodstream pathogens under physiologically relevant shear stress. This will be particularly interesting in the case of bacteria that frequently cause bacteremia and bloodstream infections and that have been shown to cause thrombocytopenia but are not frequent causative agents of infective endocarditis, for example, Escherichia coli.5 It remains possible that bacterial interactions with VWF under flow are the critical discriminating factor between these different types of cardiovascular infections. This is especially relevant seeing that inflammation-activated endothelial cells appear to be a critical source reservoir of VWF that serves to capture platelet bacteria thrombi on the vessel surface.
The outstanding contribution in this paper is the real-time intravascular imaging of bacterial platelet thrombi in vivo using bacterial and mouse isogenic mutations to illustrate the conclusions drawn from the in vitro data. Encouragingly, this study shows that dabigatran and related compounds offer the possibility of adjunctive therapy to antibiotics in S aureus sepsis by slowing down or halting the coagulation of bacteria and platelets by inhibiting the action of staphylothrombin, a combination of prothrombin, S aureus coagulase (Coa), and vWbp.6 This vWbp- and Coa-mediated coagulation significantly contributes to the lethal outcomes of sepsis in vivo as observed by McAdow et al.7 However, the complications of anticoagulation therapy in sepsis represent a significant drawback in terms of bleeding risks in the clinic. Perhaps the approach taken in a 2013 study that identifies vWbp and Coa as effective vaccine targets represents a more sustainable strategy8 and an indication that the function of vWbp and Coa can be inhibited to successfully treat S aureus bloodstream infections. The data presented in this paper are a significant step in our understanding of S aureus bloodstream and vascular infections and offer a real opportunity to develop a therapeutic treatment of these infections that may have an important clinical impact and one that does not rely solely on antibiotic therapies.
Conflict-of-interest disclosure: The author declares no competing financial interests.