Key Points
DC-specific ablation of p14 leads to the disruption of the LC network in situ by inducing apoptosis and proliferation deficiency in LCs.
p14 deficiency affects ERK/mTOR signaling in DCs and results in transient recruitment of circulation-derived short-term LCs to the skin.
Abstract
Langerhans cells (LCs) are dendritic cells (DCs) residing in epithelia, where they critically regulate immunity and tolerance. The p14 adaptor molecule is part of the late endosomal/LAMTOR (lysosomal adaptor and mitogen-activated protein kinase and mammalian target of rapamycin [mTOR] activator/regulator) complex, thereby contributing to the signal transduction of the extracellular signaling-regulated kinase (ERK) and the mTOR cascade. Furthermore, p14 represents an important regulator for endosomal sorting processes within the cell. Mutated, dysfunctional p14 leads to a human immunodeficiency disorder with endosomal/lysosomal defects in immune cells. Because p14 participates in the regulation of endosomal trafficking, growth factor signaling, and cell proliferation, we investigated the role of p14 in mouse DCs/LCs using a conditional knockout mouse model. p14-deficient animals displayed a virtually complete loss of LCs in the epidermis early after birth due to impaired proliferation and increased apoptosis of LCs. Repopulation analysis after application of contact sensitizer leads to the recruitment of a transient LC population, predominantly consisting of short-term LCs. The underlying molecular mechanism involves the p14-mediated disruption of the LAMTOR complex which results in the malfunction of both ERK and mTOR signal pathways. Hence, we conclude that p14 acts as a novel and essential regulator of LC homeostasis in vivo.
Introduction
Recently, a hitherto unknown immunodeficiency disorder was discovered in the offspring of a Mennonite family.1 The clinical phenotype of this disorder included partial immunodeficiency, reminiscent of diseases associated with defects in the lysosomal pathway of cells like Chédiak-Higashi2,3 or Hermansky-Pudlak4,5 syndrome. The patients harbored CD8+ T lymphocytes with reduced cytotoxic activity and neutrophils displaying a decreased capacity to eliminate bacteria. Genetic linkage analyses disclosed a point mutation in the gene encoding for the adaptor protein p14 as the cause of this disease.1
The p14 molecule (LAMTOR2 [lysosomal adaptor and mitogen-activated protein kinase (MAPK) and mammalian target of rapamycin (mTOR) activator/regulator 2]) is part of the LAMTOR complex, consisting of p18 (LAMTOR1), p14 (LAMTOR2), MP1 (LAMTOR3), HPXIP (LAMTOR4), and C7orf59 (LAMTOR5). This complex represents a platform for the recruitment and spatiotemporal activation of the extracellular signaling-regulated kinase (ERK1/2) and the mTOR complex 1 (mTORC1).6-11
Furthermore, p14 critically participates in the regulation of endosomal trafficking, growth factor signaling (eg, epidermal growth factor [EGF] receptor), and cell proliferation.12-14 The role of p14 in such fundamental cellular and immunologic processes1,14 raised our interest to elucidate its function in dendritic cells (DCs), the key antigen-presenting cells of the mammalian organism.15
The skin represents a major entry site for pathogens as well as a target organ for vaccine delivery. We therefore studied p14 in epidermal Langerhans cells (LCs). LCs reside in the epidermis and other epithelia of the mammalian organism, representing the “first line of defense” upon encounter of invading pathogens. They are specialized for incorporation and processing of antigen, followed by migration to the skin-draining lymph nodes (LNs) to present major histocompatibility complex (MHC)-bound peptides to T lymphocytes for the purpose of generating immunity or tolerance.16-18
The immunologic importance of skin DCs, foremost LCs, and the pivotal functions of p14 in fundamental cellular processes prompted us to dissect its unknown role in LCs.
Methods
Mice
We used Langerin enhanced green fluorescent protein (EGFP),19 Langerin diphtheria toxin receptor (DTR),19 CD11c-Cre,20 Langerin-Cre,21 p14-flox,12 and Rosa26-tdTomato mice. Langerin/CD11c-Cre mice were crossed to p14-flox mice to generate LC/DC-specific p14 knockouts, respectively (Langerin/CD11c-Cre/p14flox/flox) and were analyzed until the age of 2 months. Mice were bred at the animal facility of the Department of Dermatology and Venereology. All experimental protocols were approved by the Austrian Federal Ministry of Science and Research and performed according to institutional guidelines.
Immunoblots
Cell lysates were separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis and transferred to a semi-dry chamber onto polyvinylidene difluoride membranes (Sigma). Membranes were probed with primary antibodies (LAMTOR1-LAMTOR5, pAKT, pp70S6K1, pERK1/2, pS6, p38, pJNK, and p-mTOR [Cell Signaling; New England Biolabs], Actin [Millipore; Merck]) in blocking buffer (3% bovine serum albumin, 1mM EDTA pH 8, 0,05% Tween 20, 6mM sodium azide pH 5.2) and incubated with the secondary antibodies (anti-mouse/anti-rabbit immunoglobulin G peroxidase antibody [Sigma]).
Cell suspensions
Trunk and ear skin were floated on 0.8% trypsin (Merck) for 15 to 45 minutes at 37°C, depending on the skin sample.22 For total skin digestion, whole-body wall skin was cut into small pieces and transferred into Hanks medium (without Ca2+, Mg2+; Biochrom AG), supplemented with 0.15 mg/mL Liberase (Roche) and 0.12 mg/mL DNAse I (Roche) for 1 hour at 37°C.
Statistics
Data were analyzed with the unpaired Student t test, or 1- or 2-way analysis of variance with a post-hoc test (Bonferroni or Tukey test). P values < .05 were considered as significant (*), <.01 very significant (**), and <.001 highly significant (***). Statistics were performed using PRISM 5.0 (Graphpad software).
Details of additional methods are available as supplemental Methods (see the supplemental Methods link of the online article).
Results
CD11c-specific depletion of p14 results in loss of LCs
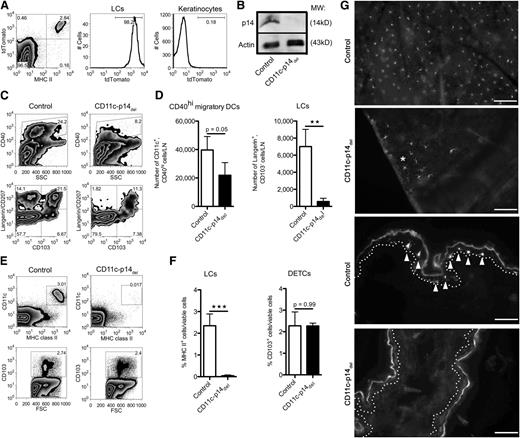
We crossed mice, whose p14 locus was flanked by loxP signal sites (p14-flox mice)12 with CD11c-Cre BAC transgenics,20 resulting in Cre-mediated deletion of the p14 gene under the control of the CD11c promoter (CD11c-p14del). As controls, we used heterozygous p14wt/flox mice (control mice), which were indistinguishable from wild type. To verify the specificity of the knockout system, we crossed p14-flox mice with a reporter mouse, expressing the molecule tdTomato under control of the Rosa26 locus,23 regulated by a loxP signal-flanked STOP cassette. Flow cytometry analysis of epidermal cell suspensions revealed specific expression of Cre in all MHC class II+ LCs, as visualized by fluorescence of the tdTomato reporter molecule, whereas MHC IIneg keratinocytes did not (Figure 1A). Western blot analyses with isolated splenic DCs ascertained the efficient ablation of the p14 molecule: p14 was completely absent in CD11c-p14del–derived DCs as compared with DCs from control mice (Figure 1B).
Adult CD11c-p14delmice lack epidermal LCs in the skin and draining LNs. (A) LC-specific expression of Cre in the epidermis. Epidermal cells derived from CD11c-Cre/p14wt/fl (control) mice, crossed to Rosa26-tdTomato reporter mice, were analyzed for the expression of the reporter molecule tdTomato in LCs (MHC II+) and keratinocytes (MHC IIneg). One representative mouse of 3 is depicted. (B) p14 protein expression in splenic DCs obtained from CD11c-p14del and heterozygous control mice. One representative of 2 experiments is shown (n = 2 mice per group). (C-D) Analysis of migratory DCs in the skin-draining LNs. Cells were pre-gated for viable CD11c+ cells. One representative mouse of 4 is shown in panel C; combined data from at least 4 individually analyzed mice per genotype are given in panel D. (E-F) Epidermal cells from adult CD11c-p14del and control mice were analyzed for the presence of LCs (CD11c+MHC II+) and DETCs (here identified by their CD103 expression). One representative of 4 mice in panel E; combined data from 4 individually analyzed mice per genotype in panel F. (G) Immunofluorescence staining of epidermal sheets (2 top panels) and cryostat sections of whole ear skin (two bottom panels) prepared from CD11c-p14del and control mice. LCs were stained for MHC II (arrows, basal lamina: dotted line). CD11c-p14del mice completely lack the LC network, except for very few residual LCs (*Patch of LCs). Scale bar for epidermal sheets: 100 µm; for sections: 50 µm. *P < .05; **P < .01; ***P < .001.
Adult CD11c-p14delmice lack epidermal LCs in the skin and draining LNs. (A) LC-specific expression of Cre in the epidermis. Epidermal cells derived from CD11c-Cre/p14wt/fl (control) mice, crossed to Rosa26-tdTomato reporter mice, were analyzed for the expression of the reporter molecule tdTomato in LCs (MHC II+) and keratinocytes (MHC IIneg). One representative mouse of 3 is depicted. (B) p14 protein expression in splenic DCs obtained from CD11c-p14del and heterozygous control mice. One representative of 2 experiments is shown (n = 2 mice per group). (C-D) Analysis of migratory DCs in the skin-draining LNs. Cells were pre-gated for viable CD11c+ cells. One representative mouse of 4 is shown in panel C; combined data from at least 4 individually analyzed mice per genotype are given in panel D. (E-F) Epidermal cells from adult CD11c-p14del and control mice were analyzed for the presence of LCs (CD11c+MHC II+) and DETCs (here identified by their CD103 expression). One representative of 4 mice in panel E; combined data from 4 individually analyzed mice per genotype in panel F. (G) Immunofluorescence staining of epidermal sheets (2 top panels) and cryostat sections of whole ear skin (two bottom panels) prepared from CD11c-p14del and control mice. LCs were stained for MHC II (arrows, basal lamina: dotted line). CD11c-p14del mice completely lack the LC network, except for very few residual LCs (*Patch of LCs). Scale bar for epidermal sheets: 100 µm; for sections: 50 µm. *P < .05; **P < .01; ***P < .001.
To assess effects of p14 deletion on DCs, we analyzed skin-draining LNs of adult CD11c-p14del and control animals. CD11c-p14del mice showed markedly reduced numbers of skin-derived CD11c+CD40high DCs (Figure 1C-D). Based on the expression of langerin and CD103, we further separated the CD40high migratory DC population into epidermal LCs (langerin+CD103neg), langerin+ dermal DCs (langerin+CD103+), and langerinneg dermal DCs (langerinnegCD103+/neg).17 Both langerin+ DC subsets were significantly reduced in CD11c-p14del mice, especially epidermal LCs (Figure 1C-D). Analysis of LN-resident DC subsets, as well as plasmacytoid DCs in the LNs of up to 6-week-old mice did not show any significant differences between CD11c-p14del and control mice (supplemental Figure 1A-B). The loss of LCs in the skin-draining LNs corresponded to an almost complete absence of LCs in the epidermis of adult CD11c-p14del mice (Figure 1E-G; supplemental Figure 1C). Only in rare cases could we detect LCs, which remained in patches of 10 to 20 cells, displaying a phenotype featuring some maturation markers like increased size and reduced number of dendrites (Figure 1G asterisk). No differences were found in the percentages of CD103+ dendritic epidermal T cells (DETCs) that were analyzed in parallel (Figure 1E-F).
Loss of LCs is due to LC-intrinsic depletion of p14
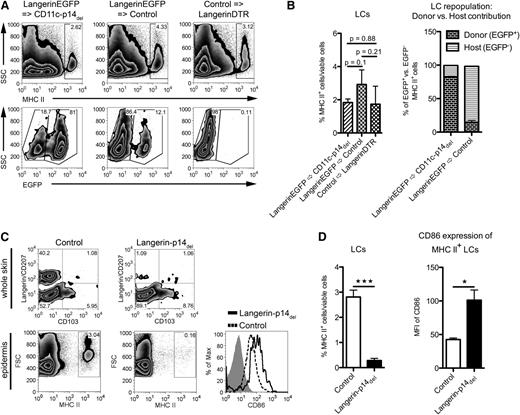
To determine whether the absence of LCs in CD11c-p14del mice is based on cell-intrinsic, LC-specific deletion of p14, or depends on extrinsic factors, we carried out bone marrow (BM) transfer experiments to investigate whether p14-sufficient LCs are capable of repopulating the epidermis of adult CD11c-p14del mice. Donor BM was derived from LangerinEGFP19 as well as control mice.
For testing of the LC repopulation, we reconstituted LC-depleted LangerinDTR mice19 (by injection of diphtheria toxin) with BM from LangerinEGFP mice and analyzed LC repopulation at defined time points after BM transfer by intravital microscopy of the epidermis (supplemental Figure 2A). Whereas 3 to 7 weeks after BM transfer only very few dendritic, EGFP+ cells were scattered across the epidermis (supplemental Figures 2B and 3; supplemental Video), by 13 weeks sizeable numbers of EGFP+ LCs were present in the epidermis (supplemental Figures 2B and 4; supplemental Video). These data demonstrate that repopulation of donor BM-derived LCs had taken place in the absence of inflammation.
To assess, whether the repopulated LCs remain in the epidermis, we finally analyzed the skin 20 weeks after BM transfer. We detected a similar MHC II+ LC population in the epidermis of both LC-free CD11c-p14del and LC-depleted LangerinDTR mice, reconstituted with LangerinEGFP or control BM, respectively. The origin of LCs in recipient animals was determined by their EGFP expression. Reconstituted control mice displayed an unchanged number of LCs because the cells had not been depleted due to their radioresistance.24 Thus, the major fraction (85.8% ± 7.6% SD) of the LCs were host-derived EGFPneg. LCs. In contrast, the majority of LCs found in CD11c-p14del mice were EGFP+ (82.2% ± 1.1% SD), and thus donor-derived (Figure 2A-B). These donor:host ratios were confirmed among langerin+CD103neg immigrant LCs in the skin-draining LNs (supplemental Figure 2C). These data indicate that p14-sufficient LCs are able to colonize the epidermis of CD11c-p14del mice.
Loss of LCs is due to the LC-intrinsic ablation of p14. (A-B) Analysis of epidermal cells for repopulation of LCs 20 weeks after BM transfer. Reconstitution of CD11c-p14del mice with BM from LangerinEGFP mice results in a MHC II+ LC population consisting mainly of donor EGFP+ BM cells (LangerinEGFP→CD11c-p14del). This is comparable to the control approach, in which BM from control mice was transferred into LC-depleted LangerinDTR mice (Control→LangerinDTR). LangerinEGFP BM transfer into control mice yields an MHC II+ LC population of mostly recipient origin, that is, EGFPneg (LangerinEGFP→Control). One representative mouse for each BM chimera in panel A (n = 2); combined data from 3 individually analyzed mice per group in panel B. (C-D) Analysis of adult Langerin-p14del and control mice for the presence and maturation status of LCs in whole skin (pre-gated for viable CD11c+ cells) and epidermis (gated for viable cells only). Histogram: isotype, gray filled; control, dotted line; Langerin-p14del, black line. One representative mouse in panel C (n = 3); combined data (corresponding to epidermal cell analysis) from 4 individually analyzed mice per genotype in panel D. *P < .05, **P < .01, ***P < .001.
Loss of LCs is due to the LC-intrinsic ablation of p14. (A-B) Analysis of epidermal cells for repopulation of LCs 20 weeks after BM transfer. Reconstitution of CD11c-p14del mice with BM from LangerinEGFP mice results in a MHC II+ LC population consisting mainly of donor EGFP+ BM cells (LangerinEGFP→CD11c-p14del). This is comparable to the control approach, in which BM from control mice was transferred into LC-depleted LangerinDTR mice (Control→LangerinDTR). LangerinEGFP BM transfer into control mice yields an MHC II+ LC population of mostly recipient origin, that is, EGFPneg (LangerinEGFP→Control). One representative mouse for each BM chimera in panel A (n = 2); combined data from 3 individually analyzed mice per group in panel B. (C-D) Analysis of adult Langerin-p14del and control mice for the presence and maturation status of LCs in whole skin (pre-gated for viable CD11c+ cells) and epidermis (gated for viable cells only). Histogram: isotype, gray filled; control, dotted line; Langerin-p14del, black line. One representative mouse in panel C (n = 3); combined data (corresponding to epidermal cell analysis) from 4 individually analyzed mice per genotype in panel D. *P < .05, **P < .01, ***P < .001.
To further test the hypothesis that the loss of LCs is directly dependent on the deletion of p14 within LCs, we crossed the p14-flox mutation to Langerin-Cre mice.21 This narrowed down the p14 deficiency to langerin+ cells within the DC population. Similar to adult CD11c-p14del mice, Langerin-p14del selectively lacked LCs (Figure 2C-D). Moreover, the few remaining LCs displayed phenotypical features of maturation such as enhanced expression of CD86 (Figure 2C-D), supporting the initial morphologic observation of the few remaining LCs in the CD11c-p14del mice (Figure 1G asterisk).
We conclude that the loss of LCs in both CD11c-p14del and Langerin-p14del mice is due to cell-intrinsic effects caused by deletion of p14, rather than to secondary changes in their local environment.
CD11c-specific depletion of p14 leads to disruption of the LC network soon after birth
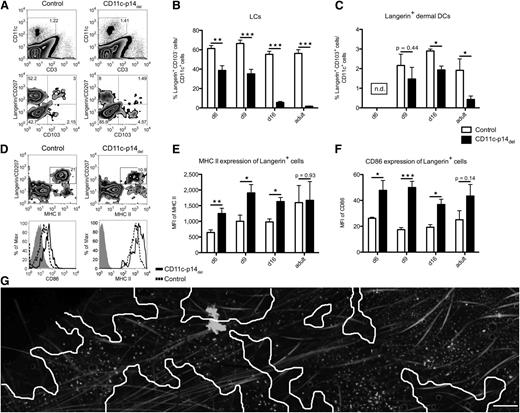
LC deficiency in adult mice does not reveal whether LCs are still able to populate the epidermis of newborn CD11c-p14del mice during ontogeny. To this end, we analyzed the skin of mice at defined time points after birth. Although we did not notice any difference in the number of LCs colonizing the epidermis on the day of birth and on postnatal day 3 (data not shown), we observed a continuous decline in the percentage of LCs (langerin+CD103neg) starting around day 6 after birth (Figure 3A-B). This decline was also seen for langerin+ dermal DCs (langerin+CD103+) (Figure 3A,C). The loss of langerin+ LCs/DCs from the skin of CD11c-p14del mice was accompanied by elevated MHC class II and CD86 expression, suggesting increased maturation of the cells (Figure 3D-F). When we analyzed LCs by fluorescence microscopy, the integrity of the initial LC network in neonatal CD11c-p14del mice was severely impaired at later time points. On postnatal day 16, large epidermal areas devoid of LCs became apparent. The remaining LCs showed signs of maturation (enlarged cell body and MHC IIbright), similar to the few remaining LCs in adult CD11c-p14del and Langerin-p14del mice (Figure 3G).
CD11c-specific depletion of p14 leads to a decrease of LCs in neonatal mice and maturation of langerin+cells. (A-B) Analysis of neonatal whole skin at defined time points after birth. Cells were pre-gated on viable CD11c+ cells. One representative experiment of a 16-day-old neonatal mouse in panel A (n = 5); combined data from at least 6 individually analyzed mice per genotype in panels B and C. (D-F) Increased maturation of p14-deficient, langerin+ skin DCs. Whole-skin cells were pre-gated for viable CD11c+ cells. Histograms show the expression of CD86 and MHC II on langerin+ cells (isotype, gray filled; control, dotted line; CD11c-p14del, black line). One representative experiment of a 9-day-old neonatal mouse in panel D (n = 5); combined data from 5 individually analyzed mice per genotype in panels E and F. (G) MHC II immunofluorescence staining of epidermal sheets of a 16-day-old CD11c-p14del mouse, illustrating the disrupted LC network. Skin areas devoid of LCs are indicated with white lines. Scale bar: 200 µm. *P < .05, **P < .01, ***P < .001.
CD11c-specific depletion of p14 leads to a decrease of LCs in neonatal mice and maturation of langerin+cells. (A-B) Analysis of neonatal whole skin at defined time points after birth. Cells were pre-gated on viable CD11c+ cells. One representative experiment of a 16-day-old neonatal mouse in panel A (n = 5); combined data from at least 6 individually analyzed mice per genotype in panels B and C. (D-F) Increased maturation of p14-deficient, langerin+ skin DCs. Whole-skin cells were pre-gated for viable CD11c+ cells. Histograms show the expression of CD86 and MHC II on langerin+ cells (isotype, gray filled; control, dotted line; CD11c-p14del, black line). One representative experiment of a 9-day-old neonatal mouse in panel D (n = 5); combined data from 5 individually analyzed mice per genotype in panels E and F. (G) MHC II immunofluorescence staining of epidermal sheets of a 16-day-old CD11c-p14del mouse, illustrating the disrupted LC network. Skin areas devoid of LCs are indicated with white lines. Scale bar: 200 µm. *P < .05, **P < .01, ***P < .001.
First mechanism causing disruption of the LC network in neonatal CD11c-p14del mice: increased LC apoptosis
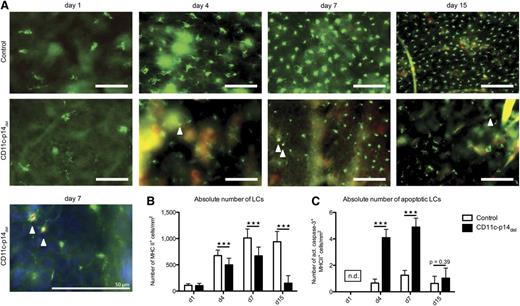
Next, we sought to determine the mechanism of the incessant decline of LCs in CD11c-p14del mice. Apoptosis being a likely cause, we analyzed LCs in situ for expression of active caspase-3 at defined time points after birth. In agreement with the flow cytometry data (Figure 3A-B), numbers of LCs per mm2 were not divergent at birth but declined in CD11c-p14del mice within 1 week after birth (Figure 4A-B). More importantly, we noticed increased absolute numbers of active caspase-3+ LCs per mm2 at postnatal days 4 and 7 in CD11c-p14del mice, indicating that apoptosis contributes to the loss of LCs (Figure 4C). No apoptotic LCs were detectable on day 1 and 15 after birth, due to the very low numbers of LCs. These data indicate that loss of p14-deficient LCs is at least partially due to increased apoptosis.
CD11c-specific depletion of p14 leads to increased LC apoptosis. (A) Epidermal sheets of control and CD11c-p14del mice were obtained on postnatal days 1, 4, 7 from abdominal skin and on day 15 from ear skin. LCs were stained for MHC II (green fluorescence) and the apoptosis marker active caspase-3 (red fluorescence, arrowheads). Scale bar: 50 µm. (B-C) Enumeration of total and apoptotic LCs at indicated time points after birth. Fifteen pictures of randomly chosen areas were recorded and the total number of MHC II+ LCs as well as the number of MHC II and active caspase-3 double-positive apoptotic LCs per mm2 was determined. The number of apoptotic LCs on day 1 after birth could not be determined (n.d.) because too few LCs were present. Combined data from 3 individually analyzed mice per genotype and time point are presented. ***P < .001.
CD11c-specific depletion of p14 leads to increased LC apoptosis. (A) Epidermal sheets of control and CD11c-p14del mice were obtained on postnatal days 1, 4, 7 from abdominal skin and on day 15 from ear skin. LCs were stained for MHC II (green fluorescence) and the apoptosis marker active caspase-3 (red fluorescence, arrowheads). Scale bar: 50 µm. (B-C) Enumeration of total and apoptotic LCs at indicated time points after birth. Fifteen pictures of randomly chosen areas were recorded and the total number of MHC II+ LCs as well as the number of MHC II and active caspase-3 double-positive apoptotic LCs per mm2 was determined. The number of apoptotic LCs on day 1 after birth could not be determined (n.d.) because too few LCs were present. Combined data from 3 individually analyzed mice per genotype and time point are presented. ***P < .001.
To investigate whether enhanced migration might also contribute to the disappearance of LCs, we analyzed skin-draining LNs of 9-day-old mice. We hypothesized that accelerated migration of LCs should yield more CD40high migratory DCs in the LNs of CD11c-p14del mice. On the contrary, CD11c+CD40high skin-immigrant DCs, including the CD40highlangerin+ DC subpopulation, were already reduced at this time point (supplemental Figure 5A-B). Furthermore, at day 6 after birth, expression of the migration-related chemokine receptor CCR7 on isolated LCs and LCs derived from epidermal explant cultures was not significantly different between CD11c-p14del and control mice (supplemental Figure 5C-E). We therefore conclude that increased migration does not contribute to the loss of LCs in CD11c-p14del mice.
Second mechanism contributing to the disruption of the LC network in neonatal CD11c-p14del mice: impaired LC proliferation in situ
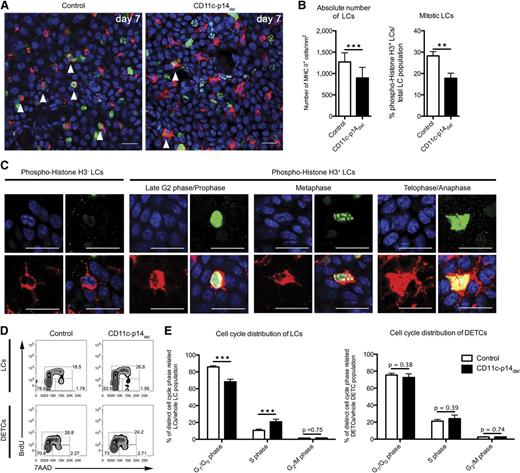
The LC network is established within the first 1 to 2 weeks after birth due to proliferation of LCs in situ.25,26 Hence, we asked whether p14 deletion in LCs affects this early wave of proliferation. To this end, we assessed LC mitosis in situ using phospho-histone H3 expression as a specific marker.27 Due to the kinetics of CD11c-mediated p14 deletion, we analyzed the skin of 7-day-old mice and observed significantly less mitotic LCs in CD11c-p14del as compared with control mice (17.7% ± 2.4% SD vs 28.2% ± 2.0% SD of all LCs) (Figure 5A-B). The nuclear staining pattern of phospho-histone H3 allowed determination of the mitotic phase at the time point of analysis. In both mice, LCs were found in late G2 phase/mitotic prophase, metaphase, and ana/telophase (Figure 5C). However, the low numbers of LCs in the different mitotic phases prevented a reliable comparative quantification.
CD11c-specific depletion of p14 inhibits mitosis of LCs. (A-B) Quantification of total and mitotic LCs of 7-day-old CD11c-p14del and control mice. LCs are stained for MHC II (red fluorescence) and phospho-Histone H3 (green fluorescence). Fifteen pictures of randomly chosen areas were recorded and the total number of LCs per mm2, as well as the proportions of mitotic LCs (arrowheads), were quantified. Combined data from 3 to 4 mice per genotype are depicted in panel B. Scale bar: 20 µm. (C) Staining patterns of phospho-Histone H3 allow identification of mitotic phases: late G2 or prophase, metaphase, and telophase/cytokinesis. All stages of mitotic LCs were found both in CD11c-p14del and in control mice. Examples shown here are from control mice. (D-E) LC cell-cycle distribution based on BrdU/7AAD incorporation on day 8 after birth. MHC II+ LCs and CD3+ DETCs were analyzed for intercalation of BrdU and 7AAD and further divided into 3 cell-cycle phases: G1/G0: BrdUneg7AADlow; S: BrdU+7AADlow-high; G2/M: BrdUneg7AADhigh. One representative experiment of an 8-day-old neonatal mouse in panel D (n = 5); combined data from 8 individually analyzed mice per genotype in panel E. **P < .01, ***P < .001.
CD11c-specific depletion of p14 inhibits mitosis of LCs. (A-B) Quantification of total and mitotic LCs of 7-day-old CD11c-p14del and control mice. LCs are stained for MHC II (red fluorescence) and phospho-Histone H3 (green fluorescence). Fifteen pictures of randomly chosen areas were recorded and the total number of LCs per mm2, as well as the proportions of mitotic LCs (arrowheads), were quantified. Combined data from 3 to 4 mice per genotype are depicted in panel B. Scale bar: 20 µm. (C) Staining patterns of phospho-Histone H3 allow identification of mitotic phases: late G2 or prophase, metaphase, and telophase/cytokinesis. All stages of mitotic LCs were found both in CD11c-p14del and in control mice. Examples shown here are from control mice. (D-E) LC cell-cycle distribution based on BrdU/7AAD incorporation on day 8 after birth. MHC II+ LCs and CD3+ DETCs were analyzed for intercalation of BrdU and 7AAD and further divided into 3 cell-cycle phases: G1/G0: BrdUneg7AADlow; S: BrdU+7AADlow-high; G2/M: BrdUneg7AADhigh. One representative experiment of an 8-day-old neonatal mouse in panel D (n = 5); combined data from 8 individually analyzed mice per genotype in panel E. **P < .01, ***P < .001.
Additionally, we carried out 5-bromo-2′-deoxyuridine (BrdU) assays to quantify LC cell-cycle distribution by flow cytometry over a short period of time (∼16 hours). To this end, 7-day-old CD11c-p14del and control mice were treated with BrdU by subcutaneous injection 16 and 2 hours prior to the experiment, and epidermal cell suspensions from BrdU-injected skin areas were analyzed on postnatal day 8. Proportions of cells residing in the 3 cell-cycle phases (G1/G0, S, G2/M) were determined according to BrdU/7-Aminoactinomycin D (7AAD) intercalation (supplemental Figure 6). At first glance, we observed a shift from G1/G0 to S phase in LCs from CD11c-p14del mice (Figure 5D-E), suggesting a higher proliferation rate. However, no corresponding increase in the percentage and number of LCs was detected at any time point (Figures 4B and 5B), making hyperproliferation of CD11c-p14del LCs unlikely. Instead, the accumulation of LCs in S-phase after 16 hours of BrdU treatment, combined with the reduced percentage of mitotic LCs observed in situ, points to a cell-cycle arrest and a failure to enter mitosis of p14-deficient LCs. As expected, there was no difference in cell-cycle distribution of DETCs that were analyzed in parallel as an internal control (Figure 5D-E).
Taken together, p14-deficient LCs cannot enter mitosis, as is evident from the significantly reduced number of phospho-histone H3+ LCs. Thus, we conclude that p14 critically regulates the initial, postnatal proliferation of LCs.
Skin inflammation in p14-deficient mice leads to a transient repopulation of the epidermis by short-term LCs
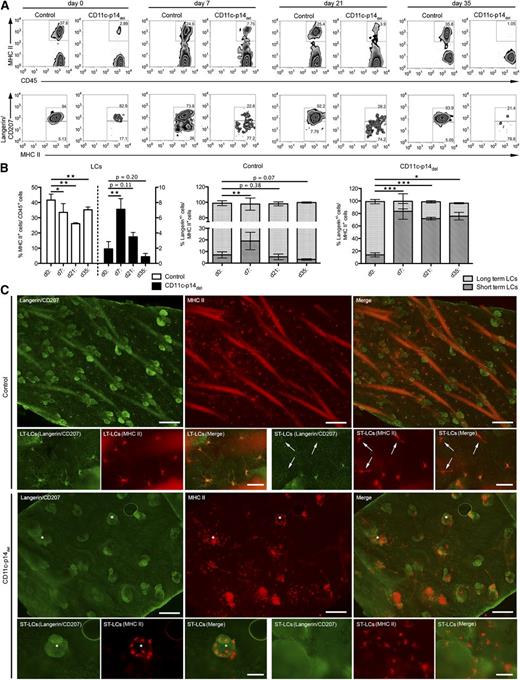
We could not detect any repopulation by LCs in steady state in CD11c-p14del mice. Hence, we asked whether LC repopulation can be induced under inflammatory conditions elicited by topical application of the contact sensitizer trinitrochlorobenzene (TNCB). Recently, it was shown that repopulation of LCs under inflammatory condition is accomplished by 2 waves of LC populations. The first and minor population, consisting of transient short-term LCs (MHC II+langerinlow/neg), differentiated from circulating monocytes, and a second major population, representing persistent long-term LCs (ie, steady-state LCs, MHC II+langerin+),28,29 presumably originated from CD11c+ pre-DCs. As previously described,30 TNCB treatment led to a gradual loss of LCs in the epidermis of control mice. In contrast, CD11c-p14del mice, that have virtually no LCs right from the start of the experiment, displayed a significant increase in the percentage of MHC II+ cells 1 week after TNCB application. However, the recruitment of MHC II+ cells in CD11c-p14del animals was only transient, as the cell numbers dropped again at later time points (Figure 6A-B). To distinguish short-term from long-term LCs, we analyzed the langerin expression of MHC II+ cells. In control mice, a significant increase of langerinlow/neg. short-term LCs was observed, despite the gradual loss of langerin+ long term-LCs, that is, steady-state LCs, 7 days after TNCB treatment. These short-term LCs were replaced by long-term LCs at later time points (21 and 35 days after treatment). Interestingly, in CD11-p14del mice, TNCB treatment led to the recruitment of langerinlow/neg short-term but not long-term LCs, which explains the transient repopulation by MHC II+ cells in these mice, as short-term LCs have been shown to decline approximately 1 to 2 weeks after the inflammatory trigger was set.28,29 As previously described,28 we observed that short-term LCs in CD11c-p14del mice entered the skin predominantly via hair follicles (Figure 6C asterisk). Surprisingly, TNCB-induced LC repopulation in Langerin-p14del mice led to the recruitment of short-term as well as long-term LCs (supplemental Figure 7A-B), suggesting that the failure of CD11c-p14del mice to recruit long-term LCs might be due to premature depletion of p14 in CD11c+ pre-DCs already on their way to the epidermis. Additionally, we noticed different expression levels of CD11c between long-term and short-term LCs, the latter displaying significantly reduced levels (supplemental Figure 7C-D).
CD11c-specific depletion of p14 leads to the recruitment of short-term LCs but not long-term LCs to inflamed skin. (A-B) Analysis of the total LC population (MHC II+ cells in the epidermus) on day 0 (ie, untreated) as well as 7, 21, and 35 days after TNCB treatment. The proportions of short-term LCs (MHC II+ langerinneg cells) and long-term LCs (MHC II+ langerin+ cells) were determined for each time point. One representative experiment of each genotype and time point is shown in panel A; combined data from at least 4 individually analyzed mice per genotype and time point in panel B. (C) Immunofluorescence microscopy analysis of long-term– and short-term–LCs (arrows) in situ, 7 days after TNCB treatment. LCs were stained for langerin (green fluorescence) and MHC II (red fluorescence). *Hair follicles. One representative mouse of 3 mice per genotype is shown in panel C. Scale bar: 200 µm for lower magnification, 50 µm for higher magnification pictures. *P < .05, **P < .01, ***P < .001. LT-LC, long-term LC; ST-LC, short-term LC.
CD11c-specific depletion of p14 leads to the recruitment of short-term LCs but not long-term LCs to inflamed skin. (A-B) Analysis of the total LC population (MHC II+ cells in the epidermus) on day 0 (ie, untreated) as well as 7, 21, and 35 days after TNCB treatment. The proportions of short-term LCs (MHC II+ langerinneg cells) and long-term LCs (MHC II+ langerin+ cells) were determined for each time point. One representative experiment of each genotype and time point is shown in panel A; combined data from at least 4 individually analyzed mice per genotype and time point in panel B. (C) Immunofluorescence microscopy analysis of long-term– and short-term–LCs (arrows) in situ, 7 days after TNCB treatment. LCs were stained for langerin (green fluorescence) and MHC II (red fluorescence). *Hair follicles. One representative mouse of 3 mice per genotype is shown in panel C. Scale bar: 200 µm for lower magnification, 50 µm for higher magnification pictures. *P < .05, **P < .01, ***P < .001. LT-LC, long-term LC; ST-LC, short-term LC.
In summary, these data indicate that TNCB-mediated inflammation leads to a transient recruitment of MHC II+langerinneg/low short-term LCs in CD11c-p14del mice. Permanent repopulation did not occur.
Depletion of p14 impairs mTORC1 and ERK signaling in DCs
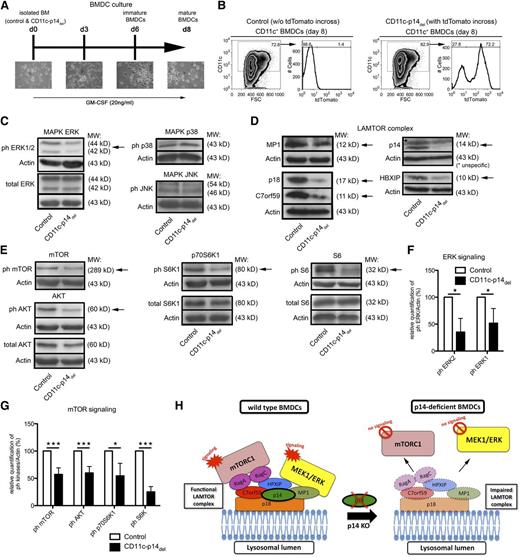
We sought to gain insight into the molecular mechanisms underlying the observed effects of p14 deletion on LC homeostasis. It is, however, extremely difficult to isolate sufficient amounts of highly purified LCs from the skin of newborn mice,25 particularly from the declining LC pool in CD11c-p14del mice. Therefore, we turned to BM-derived DCs (BMDCs) obtained from CD11c-p14del and control mice (Figure 7A). To evaluate the efficiency of CD11c-Cre–mediated p14 deletion, we analyzed BMDCs differentiated from CD11c-p14del/R26-tdTom mice for expression of the tdTomato reporter gene. About 70% to 80% of CD11c+ BMDCs were positive for tdTomato and were thus considered to have deleted p14 (Figure 7B). Based on previous reports showing that p14 depletion affects MAPK signaling in nonimmune12 and immune cells,14 we analyzed the activation of the classical MAPK effector kinases ERK1/2, c-Jun N-terminal kinase (JNK), and p38. We noticed significantly decreased activity of ERK1 and 2 in p14-deficient BMDCs. Activity of JNK and p38 in p14-deficient BMDCs appeared to be similar to control BMDCs (Figure 7C,F). p14 is part of the LAMTOR complex, consisting of p18 (LAMTOR1), p14 (LAMTOR2), MP1 (LAMTOR3), and 2 recently described molecules called HPXIP (LAMTOR4) and C7orf59 (LAMTOR5).9 The LAMTOR complex is responsible for the recruitment and activation of ERK and mTORC1 to the lysosomal membrane. All 5 LAMTOR complex components were expressed at reduced levels in CD11c-p14del BMDCs (Figure 7D).9,31 Furthermore, the phosphorylation status of AKT, mTOR, p70S6K1, and S6, all participating in mTORC1 signaling, was significantly reduced indicating decreased activation in p14-deficient BMDCs compared with control BMDCs (Figure 7E,G).
CD11c-specific depletion of p14 leads to reduced expression of the LAMTOR complex and impairs ERK and mTORC1 signaling. (A) Scheme of BMDC culture, derived from CD11c-p14del and control mice. (B) Analysis of the percentage of CD11c+ BMDCs as well as their tdTomato expression on day 8 of CD11c-p14del and control BM culture. One representative experiment of 2 is shown. (C,F) Analysis of phosphorylation and subsequent quantification of the MAPK effector kinases ERK1 and 2, JNK, and p38 in CD11c+ BMDCs derived from CD11c-p14del and control BMDCs. (D) Expression analysis of LAMTOR complex proteins: p18, MP1, p14, HPXIP, and C7orf59 in CD11c+ BMDCs derived from CD11c-p14del and control BMDCs. (E-F) Analysis of phosphorylation and subsequent quantification of the mTOR pathway related molecules AKT, mTOR, p70S6K1, and S6 kinase in CD11c+ BMDCs, derived from CD11c-p14del and control BMDCs. One representative experiment of 3 is shown in panels C-E. Combined data from 3 to 4 individually analyzed mice per genotype are depicted in panels F and G. (H) Scheme of LAMTOR complex disruption caused by loss of p14, which leads to malfunction of the ERK and mTOR pathway in p14-deficient DCs. *P < .05, **P < .01, ***P < .001.
CD11c-specific depletion of p14 leads to reduced expression of the LAMTOR complex and impairs ERK and mTORC1 signaling. (A) Scheme of BMDC culture, derived from CD11c-p14del and control mice. (B) Analysis of the percentage of CD11c+ BMDCs as well as their tdTomato expression on day 8 of CD11c-p14del and control BM culture. One representative experiment of 2 is shown. (C,F) Analysis of phosphorylation and subsequent quantification of the MAPK effector kinases ERK1 and 2, JNK, and p38 in CD11c+ BMDCs derived from CD11c-p14del and control BMDCs. (D) Expression analysis of LAMTOR complex proteins: p18, MP1, p14, HPXIP, and C7orf59 in CD11c+ BMDCs derived from CD11c-p14del and control BMDCs. (E-F) Analysis of phosphorylation and subsequent quantification of the mTOR pathway related molecules AKT, mTOR, p70S6K1, and S6 kinase in CD11c+ BMDCs, derived from CD11c-p14del and control BMDCs. One representative experiment of 3 is shown in panels C-E. Combined data from 3 to 4 individually analyzed mice per genotype are depicted in panels F and G. (H) Scheme of LAMTOR complex disruption caused by loss of p14, which leads to malfunction of the ERK and mTOR pathway in p14-deficient DCs. *P < .05, **P < .01, ***P < .001.
Finally, this leads to the conclusion that p14 depletion in DCs disrupts the assembly of the LAMTOR complex thereby affecting the spatial regulation of activation of the ERK and mTORC1 signaling cascades (Figure 7H).
Discussion
The adaptor molecule p14 contributes to the formation of the LAMTOR complex at the membrane of lysosomal compartments and induces the recruitment and activation of the ERK6-8 and mTORC1 signal cascades.9,10 By using a conditional knockout mouse model, we discovered that CD11c- as well as Langerin-specific depletion of p14 (LAMTOR2) has a profound impact on the homeostasis of the LC network, reflected by the virtually complete loss of LCs in these mice. This observation was unexpected because, unlike LC deficiency caused by the lack of transforming growth factor β32,33 and the recently described interleukin-34 (IL-34),34,35 p14 affects various cellular processes in immune1,14 as well as in nonimmune cells.12 To date, there are no reports in the literature that p14 has any role in orchestrating the function of DCs. Here, we demonstrate for the first time that p14 is crucial for DC/LC homeostasis by affecting the formation of the LAMTOR complex and subsequent ERK as well as mTOR signaling.
Analyses of newborn skin from CD11c-p14del mice revealed that initial seeding of the epidermis with LC precursors within the first 2 to 3 days after birth was intact. Rather, spontaneous maturation besides defective proliferation and increased apoptosis of LCs occurred, leading to disruption of the epidermal LC network. This is in contrast to recent work on IL-34 in the development of LCs.34,35 In those studies, LCs never appear in the epidermis in the first place because IL-34 is critically involved in early LC differentiation.
At first glance our data might suggest that the homeostasis only of skin DCs, especially LCs, is dependent on p14. Indeed, when we analyzed additional DC subsets (migratory DCs) in the skin-draining LNs of up to 6-week-old CD11c-p14del mice both LN-resident DCs and plasmacytoid DCs remained unchanged in spite of p14 depletion. However, it should be mentioned that CD11c-p14del mice eventually develop an additional phenotype represented by hyperproliferation of various myeloid cell populations, including other, langerinneg DCs. Importantly, this occurs only when mice grow older (above 2 months of age), that is, beyond the age period of the here-described investigations (J.M.S., F.S., P.S., N.R., and L.A.H., manuscript in preparation). This phenotype is currently being analyzed. The 2 phenotypes observed in CD11c-p14del mice, that is, early postnatal loss of LCs (this manuscript) and late-onset myeloproliferation (J.M.S., F.S., P.S., N.R., and L.A.H., manuscript in preparation) thus concur with current knowledge that LCs and the other DC subsets are distinct in their development and growth factor requirements (reviewed in Merad et al16 ).
In the first 2 months of life, the LC population is selectively and dramatically affected by p14 depletion in CD11c-p14del; a possible reason for this may be its long half-life and extremely slow turnover.24 Even if other DC populations may also show some effects on proliferation and apoptosis as a consequence of the impairment of ERK and mTOR, this would not result in the loss of almost the entire population (as shown here for LCs) because supply with newly arriving fresh precursor cells from the BM is constantly operating. This would permanently replenish the population and prevent it from disappearing.
We identified the cellular mechanisms accounting for the LC ablation. First, direct in situ analysis of epidermal sheets indicated increased apoptosis of LCs. Approximately 0.1% of all LCs were apoptotic in control mice postnatally, while this proportion was up to 10 times higher in CD11c-p14del mice. Second, the postnatal LC proliferation described earlier25,26 was markedly attenuated in CD11c-p14del LCs as indicated by the reduced percentage (one-third less) of mitotic, p14-depleted LCs in situ as compared with wt cells. The accumulation of CD11c-p14del LCs in S-phase, visualized by BrdU/7AAD analysis, further emphasizes the inferiority of CD11c-p14del LCs to enter mitosis. The observation that p14 deletion affects the proliferative behavior of LCs is in agreement with reduced proliferation of keratinocytes by conditional deletion of p14 under the keratin-5 promotor.12
Throughout our analyses, we could not observe any sign of spontaneous LC repopulation in CD11c-p14del mice in situ. As recently shown, LC repopulation during inflammation, but presumably also in steady state, is accomplished by 2 waves of LCs: a minor and transient population of MHC II+langerinlow/neg short-term LCs followed by the major and persistent population of MHCII+langerin+ long-term LCs, that is, steady-state LCs.28,29 Treatment of CD11c-p14del mice with the contact sensitizer TNCB resulted in a transient recruitment of MHCII+ cells, predominantly langerinneg short-term LCs. The reason for the absence of langerin+ long-term LCs in CD11c-p14del mice might be explained by the difference in CD11c expression between short-term and long-term LCs. We show that long-term LCs express markedly higher levels of CD11c and are thus more susceptible to the p14 depletion than short-term LCs. Low CD11c expression of short-term LC is likely a trait related to their origin from circulating Gr-1high/CD11cneg monocytic cells.28,29,36 Long-term LCs, in contrast, presumably arise from CD11c+ DC precursors derived from the BM.37 Our hypothesis that p14 depletion might already affect CD11c+ DC precursors still on their way to the epidermis in CD11c-p14del mice is supported by the fact that langerin-restricted depletion of p14 in langerin-p14del mice leads to recruitment of not only short-term but also long-term LCs to the inflamed epidermis. Langerin expression and, as a consequence, p14 depletion sets in markedly later when the cells have already reached the epidermis.38
Finally, we investigated the molecular mechanism underlying the loss of LCs by means of BMDC cultures derived from CD11c-p14del and control mice. The adaptor molecule p14 has been shown to contribute to ERK as well as mTORC1 signaling in nonimmune6,9,10,12 and also immune cells.1,14 It has been shown previously that p14 depletion significantly affects protein stability of all other LAMTOR components and thereby also mTORC1 and MAPK signaling.9,31 Here, we have extended these observations toward DCs. Interestingly, a recent report showed that raptor-mediated disruption of mTORC1 signaling critically affects LC homeostasis.39 ERK as well as mTOR signaling are known to mediate/regulate fundamental cellular processes like growth factor signaling,12 cell growth and proliferation,12,40 as well as the nutritional status of the cell.9,10 This may connect cell ontogeny and mTOR/ERK signaling in that less mTORC1/ERK signaling (as shown for LCs) results in less proliferation and less inhibition (ie, promotion) of catabolic40 processes such as apoptosis, as we have established.
In summary, we demonstrate the relevance of the p14-dependent formation of the LAMTOR complex as an important regulator/activator of the ERK and mTOR pathway in LCs.
The online version of this article contains a data supplement.
There is an Inside Blood commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Dr M Sibilia (Vienna, Austria) for encouraging collaboration and B. Schwab for technical assistance.
This work was supported by grant FWF-P23548 from the Austrian Science Fund (N.R.). Additional funding was provided to P.S. (Austrian Science Fund FWF-P-21487) and to L.A.H. and N.R. (IFTZ project No.11 of Innsbruck Medical University). Work on p14 in the Huber laboratory is supported by the special research program SFB021 (FWF) “Cell proliferation and cell death in tumors.” B.E.C. is a VIDI fellow of the Netherlands Organization for Scientific Research (NWO grant 916-76-365).
Authorship
Contribution: F.S. designed, performed, and analyzed most experiments and wrote the manuscript; J.M.S. performed western blot; N.A. analyzed caspase and Histone H3 immunofluorescence experiments; C.H.T. and V.H. helped with flow cytometry; M.H. performed in vivo imaging experiments; B.R., B.E.C., and S.P.Z. provided CD11c-Cre and Langerin-Cre mice, respectively; L.A.H. provided p14-flox mice and valuable advice; and P.S. and N.R. jointly supervised the research and edited the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Nikolaus Romani, Department of Dermatology and Venereology, Innsbruck Medical University, Anichstrasse 35, A-6020 Innsbruck, Austria; e-mail: nikolaus.romani@i-med.ac.at; and Patrizia Stoitzner, Department of Dermatology and Venereology, Innsbruck Medical University, Anichstrasse 35, A-6020 Innsbruck, Austria; e-mail: patrizia.stoitzner@i-med.ac.at.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal