In this issue of Blood, Speth et al identify the beneficial effects of a pharmacologic stabilizer of hypoxia-inducible factor-1α (HIF1α) for the efficient trafficking of hematopoietic stem and progenitor cells (HSPCs) to the bone marrow niche.1
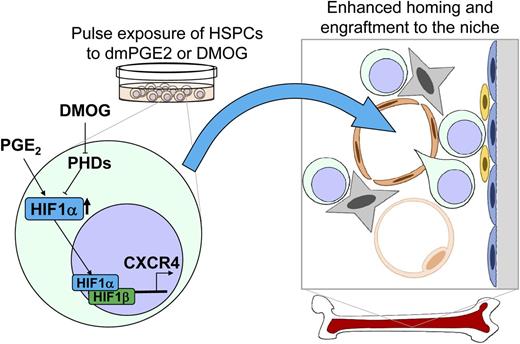
Scheme depicting the effect of HIF1α on HSPC trafficking during transplantation. Ex vivo treatment of HSPCs with PGE2 or hypoxia-inducible factor prolyl-hydroxylase inhibitor (DMOG) stabilizes HIF1α protein. Stabilized HIF1α translocates to the nucleus with the oxygen-independent subunit HIF1β to transactivate CXCR4 expression and enhance the homing and engraftment of HSPC to the niche.
Scheme depicting the effect of HIF1α on HSPC trafficking during transplantation. Ex vivo treatment of HSPCs with PGE2 or hypoxia-inducible factor prolyl-hydroxylase inhibitor (DMOG) stabilizes HIF1α protein. Stabilized HIF1α translocates to the nucleus with the oxygen-independent subunit HIF1β to transactivate CXCR4 expression and enhance the homing and engraftment of HSPC to the niche.
Bone marrow transplantation is an effective therapy for various hematologic diseases. However, insufficient numbers of transplanted hematopoietic stem cells (HSCs) can result in failure of engraftment. Thus, it has long been a dream to robustly enhance the homing efficiency of transplanted HSCs to the bone marrow microenvironment (hematopoietic niche).
Recently, a long-acting prostaglandin E2 (PGE2) analog, 16-16 dimethyl PGE2 (dmPGE2), was identified as a proliferative agent for HSPCs in a teleost chemical screening. Ex vivo treatment of dmPGE2 enhanced bone marrow engraftment of HSPCs in murine and nonhuman primate models.2,3 The report clearly showed that the PGE2-guided increase in the homing, survival, and proliferation of HSPCs was at least in part due to enhanced CXCR4 expression4 ; however, the molecular mechanisms underlying these observations remained unclear.
HSCs are maintained in a hypoxic state in which stabilization of the HIF1α protein governs cell-cycle and metabolic properties.5,6 HIF1α is known to upregulate the expression of CXCR4, an essential receptor for chemokine SDF-1 that not only enhances HSPC retention and homing to the niche but also maintains HSC cell-cycle quiescence (see figure).7
Speth et al1 found that pulse exposure of dmPGE2 stabilizes HIF1α in HSPCs. The degree of the stabilization of HIF1α is comparable to the pulse exposure of DMOG, an inhibitor of hypoxia-inducible factor prolyl hydroxylase that promotes the ubiquitin-proteasomal degradation of HIF1α protein under normoxic conditions. Pulse treatment of DMOG increased CXCR4 expression through direct transcriptional activation of a hypoxia-response element on the CXCR4 promoter and enhanced the SDF-1-dependent migration in vitro. In addition, pulse treatment of DMOG enhanced HSPC homing to the niche and improved peripheral blood chimerism after transplantation without alterations in lineage differentiation. The effect of dmPGE2 on HSPC trafficking was shown to be mediated by the HIF1α–CXCR4 pathway, because the enhanced homing and CXCR4 expression by dmPGE2 was clearly cancelled by pharmacologic HIF inhibition or genetic HIF1α deficiency.
The indication of a PGE2–HIF1α–CXCR4 axis demonstrates a crucial mechanism through which low oxygen tension affects HSC function during transplantation. Recent evidence suggests that HSCs maintain their hypoxic state independently of their bone marrow location.8 Isolation of HSPCs from their original hypoxic bone marrow microenvironment may downregulate HIF1α protein expression necessary for efficient homing during transplantation. Thus, in addition to dmPGE2 pulse exposure of HSPCs, modulation of HIF1α expression or activity may be a promising tool for better engraftment of HSCs.
Given the fact that the overstabilization of HIF1α through homozygous deletion of an E3 ubiquitin ligase for HIF1α (VHL) or a prolyl hydroxylase for HIF (Phd2) or prolonged HIF stabilizer treatment resulted in the loss of transplantation capacity of HSPCs,5,9,10 optimization of HIF1α stabilization through manipulation of these factors may be beneficial for improving human bone marrow transplantation.
The transcriptional regulation of the HIF1α expression and stabilization is still under investigation. The work by Speth et al clearly identifies that fine-tuning of the HIF1α dosage is a promising approach for improving stem cell transplantation. On the other hand, inhibition of HIF1α is an interesting approach to enhance mobilization of HSCs. Because leukemic stem cells similarly reside in the niche in a quiescent state, techniques to modulate of HIF1α levels may provide a tool for the elimination of these cells. This paper opens the door for the clinical use of HIF1α regulation.
Conflict-of-interest disclosure: The authors declare no competing financial interests.