In this issue of Blood, Rossi et al provide further evidence for clonal evolution in chronic lymphocytic leukemia (CLL) and demonstrate the clinical importance of small TP53-deleted subclones detected at diagnosis in determining the natural history of the disease.1
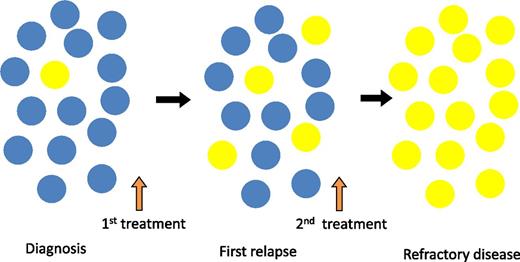
Clonal selection and expansion of TP53-mutated subclones during the clinical course of CLL. Yellow circles indicate the TP53-mutated CLL cell which expands through the disease course from below the level of conventional detection methods at diagnosis to become the dominant population in refractory disease. Blue circles indicate the non-TP53–mutated CLL cell which is the dominant clone at diagnosis but is subsequently replaced by the TP53-mutated subclone.
Clonal selection and expansion of TP53-mutated subclones during the clinical course of CLL. Yellow circles indicate the TP53-mutated CLL cell which expands through the disease course from below the level of conventional detection methods at diagnosis to become the dominant population in refractory disease. Blue circles indicate the non-TP53–mutated CLL cell which is the dominant clone at diagnosis but is subsequently replaced by the TP53-mutated subclone.
The basic principle of Darwinian evolutionary theory is the natural selection of the fittest variants. Fitness is defined by the ability to survive and reproduce and the “fittest” as those best adapted to achieve this. This concept of subclonal selection of the “fittest” variants was first applied to cancer by Nowell in 19762 and has subsequently been supported by modern genomics.3 Knowledge of the clonal diversity and clonal selection operating in any specific cancer is critical to the understanding of disease progression, response to treatment, and development of resistance. The clonal architecture of any cancer is in a constant state of evolutionary change, which can take place over prolonged periods. Clinically important mutations may be present at an early stage of the disease but only become evident over time through selective pressure.
The clinical importance of chromosomal abnormalities in CLL has been recognized since the late 1990s.4 Since then, more refined techniques such as fluorescence in situ hybridization (FISH) and Sanger sequencing have confirmed that the presence of certain genetic alterations is associated with overall prognosis and response to treatment. CLL is characterized by relatively few recurrent somatic mutations, of which those in the TP53 gene are the strongest predictors of chemoresistance and poor survival.5
In this study, Rossi et al1 have applied highly sensitive ultra-deep next-generation sequencing to examine a large cohort of patients (309) with newly diagnosed CLL for the presence of very small TP53-mutated subclones (sensitivity down to 0.3% allele frequency), which would not have been detected by Sanger sequencing (which detects >20% frequency).6 The 5.8% of patients with such subclones had the same adverse survival as those 9% in whom TP53 mutations were detected by conventional methods, and accounted for a third of all cases with TP53 abnormalities.
The current recommendation is that all patients should be tested for abnormalities of TP53 prior to initiating any line of treatment, in order to select TP53-independent therapy when appropriate. The prevailing dogma is that the size of the TP53-deleted clone is important and that below certain thresholds (variably reported as 10% or 20% using FISH), response to treatment is unaffected. Rossi et al overturn the view that small clones are clinically unimportant by showing that even very small subclones (<1%; present below the threshold of detection using current standard methods but with no apparent cutoff in the size of the clone) have an adverse impact on patient survival. They argue that the effect on outcome of the presence of these subclones is a yes/no determinant independent of clonal size.
Importantly, sequential samples showed that these subclones expanded over time, particularly under the selective pressure of chemotherapy, leading ultimately to chemorefractory disease (see figure). In the 2 patients who did not receive treatment, the clonal size remained unchanged. This would be consistent with other studies showing increasing frequency of TP53 abnormalities in patients with disease progression and refractoriness.7
If multiple subclones coexist, what drives any to become dominant? There are many reasons, including limited potential for expansion due to “competition” from other clones as well as the independent effect of the surrounding microenvironment. If these subclones are vying for space and resources, the reduction of some clones may unbalance the status quo. It is therefore unsurprising that failure of chemotherapy to completely eradicate CLL cells can result in expansion of minor, more resistant, and more dangerous subclones. “Selection” can thus be introduced artificially by the use of chemotherapeutic agents. Certain subclones are likely to gain a competitive advantage due to their “fitness” in relation to these selection pressures. It is therefore important to identify low-level molecular lesions that are known to predict for chemoresistance so that treatment can be tailored appropriately. In TP53-mutated CLL, this may involve use of novel targeted therapies (eg, Ibrutinib, ABT199)8 which have been shown to have promising activity in this subset.
What other strategies might be considered to improve therapeutic efficacy and prevent emergence of resistance? Cytotoxic drugs are likely to select for resistant cells by clearing the ground of more sensitive ones. On the other hand, cytostatic drugs (some small-molecule inhibitors) may cause cells to remain in the tissue space but without either expanding themselves or allowing expansion of other subclones. In addition, early intervention with effective treatment before clonal expansion may be a more effective way to deal with these more clinically adverse subclones. It may also be the case that carefully designed concurrent or sequential combinations of therapies may overcome some of the issues related to clonal diversity. It is important to note, however, that not all chemoresistant CLL is characterized by TP53 mutation and it will be crucial to understand the biology of any other clinically important subclones which may be present in order to prevent their dominance. The underlying principles are likely to be the same but the therapeutic strategies may be different.
Clearly, CLL is not a static disease, but has a clonal architecture that changes over time and is influenced by selection pressures, including treatment. Certain clinically adverse genomic changes appear to be present in the CLL cells from a very early stage of disease. Understanding how this population becomes dominant is crucial for the development of new therapeutic strategies, which will be effective by rendering them the least fit for survival.
Conflict-of-interest disclosure: The author declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal