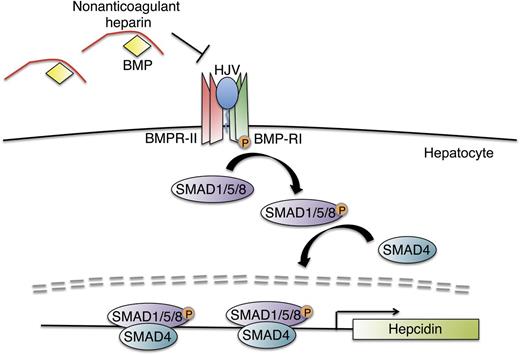
In this issue of Blood, Poli et al demonstrate that heparin analogs engineered to minimize their anticoagulant properties can potently downregulate hepcidin production in vitro and in vivo, and may potentially be used to treat the anemia of inflammation.1
Proposed role of nonanticoagulant heparins as hepcidin production inhibitors. Nonanticoagulant heparins bind to BMP ligands to prevent their interaction with BMP type I (BMP-RI) and BMP type II (BMP-RII) receptors and the coreceptor hemojuvelin on the hepatocyte membrane. Blockade of this active signaling complex inhibits phosphorylation of intracellular SMAD 1, SMAD5, and SMAD8 proteins (SMAD1/5/8) and their interaction with SMAD4, thereby blunting hepcidin transcription.
Proposed role of nonanticoagulant heparins as hepcidin production inhibitors. Nonanticoagulant heparins bind to BMP ligands to prevent their interaction with BMP type I (BMP-RI) and BMP type II (BMP-RII) receptors and the coreceptor hemojuvelin on the hepatocyte membrane. Blockade of this active signaling complex inhibits phosphorylation of intracellular SMAD 1, SMAD5, and SMAD8 proteins (SMAD1/5/8) and their interaction with SMAD4, thereby blunting hepcidin transcription.
Hepcidin is the key iron hormone that regulates iron availability from 3 main sources: dietary absorption, red blood cell recycling, and body iron stores.2 Hepcidin acts by inducing degradation of the principal iron exporter ferroportin, thereby blocking iron release into the bloodstream and leading to iron sequestration in enterocytes, macrophages, and hepatocytes.2 Increases in iron levels stimulate hepcidin production to “turn off” further iron release into the circulation and to prevent iron excess. Inflammation also stimulates hepcidin production to limit iron accessibility to infectious organisms. Alternatively, when erythropoiesis is stimulated by anemia or hypoxia, hepcidin production is inhibited to “turn on” iron release into the circulation to support red blood cell production. Diseases of dysregulated hepcidin include hemochromatosis, in which too little hepcidin leads to iron overload, and the anemia of inflammation (also called anemia of chronic disease), in which too much hepcidin leads to iron-restricted erythropoiesis.2
Hepcidin is mainly secreted by hepatocytes in the liver, where its expression is controlled by a variety of signals. One central signaling pathway that controls hepcidin production is the bone morphogenetic protein (BMP)-sons of mothers against decapentaplegic (SMAD) signaling pathway through the coreceptor hemojuvelin3 and the ligand BMP6.4 This pathway is not only essential for hepcidin regulation by iron, but its activity is also required for hepcidin activation by inflammatory cytokines via the signal transducer and activator of transcription 3 signaling pathway.5
The central function of the hepcidin- ferroportin axis in iron homeostasis regulation makes this pathway an attractive therapeutic target.6 Indeed, previous studies have demonstrated that inhibiting hepcidin production or activity with a soluble hemojuvelin fusion protein, a small-molecule BMP inhibitor, a fully humanized antihepcidin antibody, or a hepcidin-binding Spiegelmer all inhibited hepcidin expression in models of anemia of inflammation.6-8
In 2011, Poli et al discovered that heparins can block hepcidin production by inhibiting BMP-SMAD signaling, presumably by binding and sequestering BMP ligands.9 However, heparin’s anticoagulant properties pose an obvious limitation for its potential therapeutic application in the treatment of iron disorders. In the current study, Poli et al demonstrate for the first time that glycol-split, nonanticoagulant heparinoids (gs-heparins) still retain the ability to inhibit the BMP signaling pathway and prevent hepcidin production in the liver both in vitro and in vivo (see figure). Gs-heparin derivatives RO-82 and RO-68 appear to have the most potent activity in inhibiting hepcidin expression, with a 50% inhibition/inhibitory concentration of ∼120 ng/mL. The authors also show that these gs-heparins abolish hepcidin induction caused by the inflammatory cytokines interleukin-6 and Oncostatin M. Moreover, the gs-heparins blunt hepcidin induction and ameliorate hypoferremia in a lipopolysaccharide model of inflammation. Although these gs-heparins had no effect on the progression of anemia induced by a heat-killed Brucella abortus model, they did modestly improve recovery from the anemia. The majority of the antihepcidin activity of gs-heparins was attributed to their interference with the BMP-SMAD signaling pathway. This is consistent with the BMP-SMAD–dependent nature of interleukin-6–mediated induction of hepcidin expression.5
However, a number of questions remain. First, the mechanism of action of modified heparins in inhibiting hepcidin in the context of inflammation may not be completely worked out. For example, 1 of the nonanticoagulant heparins (NAc-91) had minimal impact on the BMP-SMAD signaling pathway at lower concentrations, but still inhibited hepcidin synthesis. Moreover, the nonanticoagulant heparins also appear to inhibit lipopolysaccharide induction of other inflammatory markers, including Socs3 and Crp. Indeed, it has been previously described that heparins possess anti-inflammatory activities.10 Together, these data suggest that there may be additional BMP-independent mechanisms by which heparins inhibit hepcidin expression, possibly related to their proposed anti-inflammatory activities. Moreover, issues regarding the pharmacokinetics and pharmacodynamics of the gs-heparins remain to be elucidated, as do their long-term safety profile. Obvious off-target effects of concern include residual anticoagulant activities, general inhibition of the BMP-SMAD signaling pathway, and additional nonanticoagulant activities including interaction with other growth factors.10 Nevertheless, this work shows that gs-heparins are a new class of candidate therapeutics for targeting the relevant signaling pathways that regulate hepcidin to treat the anemia of inflammation.
Conflict-of-interest disclosure: J.L.B and H.Y.L. have ownership interest in the start-up company FerruMax Pharmaceuticals, which has licensed technology from the Massachusetts General Hospital based on the work cited here and in prior publications.