In this issue of Blood, Mereuta et al report that we must now add leukocyte cell–derived chemotaxin 2 (LECT2) to the list of proteins that can cause systemic amyloidosis,1 a fibrillar protein deposition disease that leads to end-organ damage and related symptoms and requires a tissue diagnosis demonstrating apple-green birefringence in Congo red–stained sections viewed microscopically under polarized light.2 When dissected from affected tissue, digested into protein fragments of different lengths for proteomic analysis, and assessed by mass spectrometry for their original constituents, amyloid deposits reveal a unique signature of chaperones such as apolipoprotein E and serum amyloid P-component (arrows) as well as the identity of the critical main culprit: the amyloid-forming protein (red boxes).3
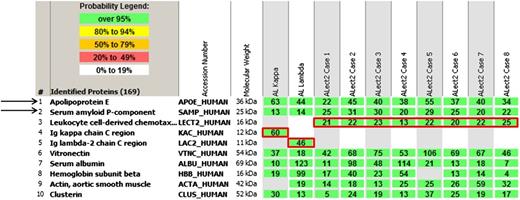
Laser microdissection/mass spectrometry read-outs of 2 cases of AL and 8 cases of ALect2 amyloidosis. The proteins identified are listed according to relative abundance based on spectral counts. The first 5 proteins represent amyloid-associated proteins identified in this cohort, followed by other abundant proteins identified in the deposits. The first 2 most abundant amyloid-associated proteins (1 and 2) across the cohort and present in every amyloid type are apolipoprotein E (APOE) and serum amyloid P-component (SAP). The following 3 proteins (3-5) highlighted by red boxes represent pathogenic proteins identified in these 10 cases. Only 1 pathogenic protein is present in each case. Rows 1 and 2 represent AL, and rows 3 to 10 ALect2. See Figure 1A in the article by Mereuta et al that begins on page 1479.
Laser microdissection/mass spectrometry read-outs of 2 cases of AL and 8 cases of ALect2 amyloidosis. The proteins identified are listed according to relative abundance based on spectral counts. The first 5 proteins represent amyloid-associated proteins identified in this cohort, followed by other abundant proteins identified in the deposits. The first 2 most abundant amyloid-associated proteins (1 and 2) across the cohort and present in every amyloid type are apolipoprotein E (APOE) and serum amyloid P-component (SAP). The following 3 proteins (3-5) highlighted by red boxes represent pathogenic proteins identified in these 10 cases. Only 1 pathogenic protein is present in each case. Rows 1 and 2 represent AL, and rows 3 to 10 ALect2. See Figure 1A in the article by Mereuta et al that begins on page 1479.
This application of mass spectrometry is most useful because the types of amyloid cannot be routinely distinguished in the clinic and because the different types are managed or treated differently.4 Common types of amyloid-forming proteins are immunoglobulin light chains, transthyretin (TTR) (both mutant and wild-type), serum amyloid A (SAA), and now LECT2. Despite our limited knowledge of LECT2-associated amyloidosis (ALect2) pathobiology, the findings Mereuta et al report immediately impact our clinical approach to certain patients with a tissue diagnosis of amyloidosis.1
The LECT2 gene has been conserved throughout vertebrate evolution and in humans is inducible in the liver in association with fatty infiltration, hepatocellular carcinoma, and regeneration. LECT2 may also participate in innate immunity.4,5 The basis for the 16-kDa LECT2 protein forming amyloid has not been studied in detail, but the presumption is that, as in inflammation-related AA amyloid due to SAA, an inducible acute-phase protein, increased production of LECT2 under certain circumstances is a predisposing factor. ALect2 amyloid has been identified in patients with hepatic and, less frequently, renal involvement, many of whom in both instances were Hispanic, a perplexing fact because no pathogenic mutations have been found, although all cases have been homozygous for a common LECT2 polymorphism. Importantly ALect2 amyloid can cause hepatic and renal failure.4,6 Whether it can cause cardiac amyloid, albeit unlikely, remains on open question.
In the series of 130 consecutive cases of hepatic biopsies with amyloid sent for mass-spectrometry typing reported by Mereuta et al, 62% (n = 81) had immunoglobulin light-chain amyloidosis (AL) and 25% (n = 32) ALect2, whereas in the 285 cases of renal biopsies with amyloid reported by Larsen et al 86% (n = 246) had AL and 2.5% (n = 7) ALect2.1,7 Of the 32 cases of ALect2 hepatic amyloid, 7 had steatosis or steatohepatitis, 5 had chronic active hepatitis (4 hepatitis C, 1 unknown), and over one-third had liver function test abnormalities; data on monoclonal gammopathies in these patients were not available from the testing center, but hepatitis C can be associated with clonal B-cell disorders, notably cryoglobulinemia.8 This point is relevant because the requirement and challenge for hematologists dealing with patients who have clonal plasma cell neoplasms and organ damage is to determine whether and how the two are linked in order to explain the illness to patients and their families and to endorse a plan of therapy.
When dealing with potential AL patients, many of whom evolve from prior monoclonal gammopathies (MG) such as monoclonal gammopathy of undetermined significance (MGUS), smoldering and symptomatic multiple myeloma (MM), or Waldenstrom macroglobulinemia (WM), the hematologist must evaluate whether the amyloid-related organ damage is due to AL or non-AL disease; that is, could the hepatic or other amyloid organ involvement be due to a culprit protein unrelated to the clonal light chain?9 Typing the amyloid, usually by mass spectrometry, is clearly necessary when patients have an MG, a tissue diagnosis of amyloid, and possibly another amyloid-forming protein. This situation may occur in patients with MG who are older men with age-related TTR cardiac amyloid or blacks with hereditary amyloid (4% prevalence of mutated TTR), as well as in patients with inflammatory disorders such as severe gout or inflammatory bowel disease and AA amyloid or in Hispanics or victims of chronic hepatitis C who have hepatic and/or renal amyloid due to LECT2. As pathologists adapt the findings of Mereuta et al to immunohistochemical staining regimens for hepatic and renal biopsy specimens containing amyloid, mass-spectrometry typing may not be required; for now, however, given its ease and reliability, it remains the gold standard for typing amyloid, particularly when there are possibly 2 amyloid-forming proteins present in the same patient.2
In the past several decades, much progress has been made in the diagnosis, prognostic evaluation, and treatment of patients with clonal plasma cell neoplasms. In fact, in practice today, we follow the principle that the genetic profile and stage of the clonal plasma cell disease and the pattern of clone-related organ damage are the axes that pose risk to patients. Just as genetically high-risk MM confers the possibilities of resistant relapse and shortened survival, AL-related visceral involvement confers the risks of organ failure and, in symptomatic cardiac patients, of shortened survival. Conversely, it must be noted that treating because of presumed AL in patients with MGUS, smoldering MM, or WM when the amyloid is in fact due to a non-AL protein confers the risk of therapy with no benefit. The advance that Mereuta et al offer us is a reminder of our promise to first do no harm and of the need, when indicated, to confirm with mass-spectrometry typing that the bad guys causing amyloid really are immunoglobulin light chains and not proteins such as TTR, SAA, or LECT2 in order “to avoid incorrect and unnecessarily toxic therapies.”
Conflict-of-interest disclosure: The author declares no competing financial interests.