Key Points
Approximately 40% of patients with undetectable minimal residual disease on imatinib can stop treatment without loss of molecular response.
Patients in treatment-free remission still have detectable BCR-ABL DNA several years after stopping imatinib.
Abstract
Most patients with chronic myeloid leukemia (CML) treated with imatinib will relapse if treatment is withdrawn. We conducted a prospective clinical trial of imatinib withdrawal in 40 chronic-phase CML patients who had sustained undetectable minimal residual disease (UMRD) by conventional quantitative polymerase chain reaction (PCR) on imatinib for at least 2 years. Patients stopped imatinib and were monitored frequently for molecular relapse. At 24 months, the actuarial estimate of stable treatment-free remission was 47.1%. Most relapses occurred within 4 months of stopping imatinib, and no relapses beyond 27 months were seen. In the 21 patients treated with interferon before imatinib, a shorter duration of interferon treatment before imatinib was significantly associated with relapse risk, as was slower achievement of UMRD after switching to imatinib. Highly sensitive patient-specific BCR-ABL DNA PCR showed persistence of the original CML clone in all patients with stable UMRD, even several years after imatinib withdrawal. No patients with molecular relapse after discontinuation have progressed or developed BCR-ABL mutations (median follow-up, 42 months). All patients who relapsed remained sensitive to imatinib re-treatment. These results confirm the safety and efficacy of a trial of imatinib withdrawal in stable UMRD with frequent, sensitive molecular monitoring and early rescue of molecular relapse.
Introduction
In the past decade, imatinib (IM) has been the standard treatment for patients with chronic-phase chronic myeloid leukemia (CML). In contrast to the previous best medical therapy (interferon alfa [IFN-α] with or without cytarabine], IM induces a major molecular response (MMR; BCR-ABL ≤0.1%) in many patients,1,2 and approximately 40% of patients who remain on IM for more than 5 years will have undetectable minimal residual disease (UMRD).3 The European LeukemiaNet management guidelines for CML currently recommend that tyrosine kinase inhibitor (TKI) therapy should be continued indefinitely in responding patients, even in those with UMRD.4
Whereas virtually all patients who stop TKI therapy with detectable MRD show rapidly rising BCR-ABL levels, this may not be true of patients who stop the TKI after a period of UMRD.5-12 The largest study to date of patients stopping IM after stable UMRD—the French STIM study6 —reported sustained treatment-free remission (TFR) in 40% of patients. Here we report similar results in a comparable group of patients in an Australasian Leukaemia & Lymphoma Group (ALLG) study that commenced in 2006. Given the potential for variability in real-time quantitative reverse-transcription polymerase chain reaction (qRT-PCR) for BCR-ABL, and the difficulty of standardizing a definition of UMRD, this independent confirmation of the STIM data in a prospective clinical trial is important. In addition, we examined a variety of clinical and laboratory parameters to identify factors that might influence the risk of relapse.
Patients and methods
The clinical trial was sponsored by the ALLG (CML8 TWISTER study, ACTRN 12606000118505) and was approved by the ethics committees of the participating hospitals. All participants gave written informed consent in accordance with the Declaration of Helsinki.
Adult CML patients were eligible if they were treated with IM and achieved UMRD during IM treatment. Prior treatment was permitted, except for allogeneic stem cell transplantation or other kinase inhibitors. Eligibility criteria included a minimum duration of UMRD of 24 months and a minimum of 36 months of IM treatment. Patients with a history of accelerated phase or blast crisis or BCR-ABL kinase domain mutations were excluded.
The eligibility criterion of sustained UMRD required no detectable BCR-ABL messenger RNA (mRNA) in any sample tested during the preceding 2 years, whether in peripheral blood (PB) or bone marrow (BM). A minimum of 2 qRT-PCR results per year were required. Thirty of the 40 patients were monitored in the central laboratory during this time, and the remaining 10 patients were monitored in their respective local laboratories. Since the sensitivity achieved in other laboratories could not be standardized retrospectively, the level of UMRD was confirmed in the central laboratory at screening with a detection limit of ≤0.0032%. An additional 6 patients were screened for eligibility but had detectable BCR-ABL mRNA despite UMRD for 2 years when tested in the central laboratory (n = 2) or elsewhere (n = 4).
Molecular monitoring was performed in the central laboratory by using PB qRT-PCR.13 In the event of relapse, qRT-PCR was performed monthly for 12 months and then every 3 months. Results were reported by using the BCR-ABL International Scale. The limit of detection of BCR-ABL in each sample was calculated by using the number of control gene transcripts.14 A calculated detection limit of 0.0032% (4.5-log) was equivalent to 400 000 BCR transcripts.3
Eligibility was confirmed by UMRD in a screening PB qRT-PCR sample. Within 1 month of screening, the patients underwent baseline assessments, including BM and PB qRT-PCR, and stopped IM. qRT-PCR was performed monthly for 12 months, every second month for 12 months, and then every 3 months during the subsequent follow-up for patients in TFR. Molecular relapse was defined as any single sample with a value of >0.1% (ie, loss of MMR) or two consecutive positive samples at any value. In the event of molecular relapse IM was recommenced at the previous effective dose, and the qRT-PCR sample was screened for BCR-ABL kinase domain mutations. The primary end point was the proportion of patients free of molecular relapse without treatment for 24 months.
To examine the value of MRD testing for prediction of relapse we performed a BM aspirate for qRT-PCR at study entry and every 3 months for the first 2 years (or until relapse) and genomic BCR-ABL PCR on selected PB samples by using patient-specific primers and real-time qRT-PCR probes, as previously described.15 These MRD studies are not routinely used for patient monitoring, and the results were not used for clinical decision-making.
Statistical analysis was performed by using SigmaPlot software. Rates of TFR were calculated by Kaplan-Meier analysis, and groups were compared by using a log-rank test. A receiver operating characteristic (ROC) curve was plotted for each continuous variable to assess predictive value for molecular relapse. Variables with an area under the curve of more than 0.5 were selected, and optimal cutoff points were determined from the ROC curve. Continuous variables that did not meet the ROC curve criterion were dichotomized about the median. P values of <.05 were considered statistically significant.
Results
Forty CML patients were enrolled in the study from August 2006 to August 2011. With a data cutoff date of December 1, 2012 the minimum follow-up was 15 months, with a median of 43 months. Patient characteristics are shown in Table 1. Nineteen patients received IM treatment de novo (IM-only cohort; short-term use of hydroxyurea prior to IM was usual), and 21 patients had received prior therapy including IFN (IFN-IM cohort). Additional prior therapies (given either as standard treatments at the time or in clinical trials) included cyclophosphamide stem cell mobilization16 (n = 2), busulphan-conditioned autologous stem cell transplantation (n = 1), low-dose cytarabine (n = 4), and all-trans retinoic acid (n = 1).
Patient characteristics at study entry
| Characteristic . | Patient cohort . | |
|---|---|---|
| IM-only (n = 19) . | IFN-IM (n = 21) . | |
| Median age (y) | 58 | 62 |
| Males (%) | 37 | 57 |
| Sokal score | ||
| High | 3 | 1 |
| Intermediate | 8 | 8 |
| Low | 8 | 12 |
| Median duration of IM (mo) | 70 | 72 |
| Median cumulative IM dose (g) | 947 | 914 |
| Median duration of stable UMRD (mo) | 30 | 41 |
| Characteristic . | Patient cohort . | |
|---|---|---|
| IM-only (n = 19) . | IFN-IM (n = 21) . | |
| Median age (y) | 58 | 62 |
| Males (%) | 37 | 57 |
| Sokal score | ||
| High | 3 | 1 |
| Intermediate | 8 | 8 |
| Low | 8 | 12 |
| Median duration of IM (mo) | 70 | 72 |
| Median cumulative IM dose (g) | 947 | 914 |
| Median duration of stable UMRD (mo) | 30 | 41 |
All comparisons between cohorts P > .05.
Relapse
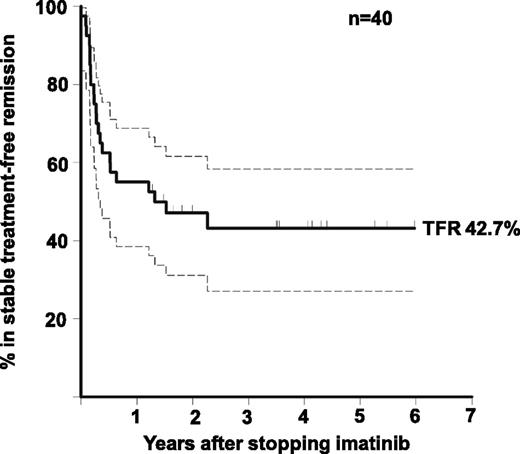
At the time of analysis 18 (45%) of 40 patients had not met the study definition of molecular relapse with a median follow-up of 42 months since IM discontinuation (range, 15 to 72 months; Figure 1). Of the 22 relapses, 15 (68%) occurred within the first 6 months after stopping IM (median, 3 months). No patient developed a kinase domain mutation, there were no deaths, and no patient progressed to advanced phase. The Kaplan-Meier estimate of the rate of TFR at 2 years was 47.1% (95% confidence interval [CI], 31.5% to 62.7%). One patient became qRT-PCR–positive between screening and study entry. In every case of molecular relapse, the first sample with detectable BCR-ABL was at a level below the MMR threshold: subsequent loss of MMR was identified in 8 patients before IM was recommenced.
Rate of TFR in all 40 patients. Actuarial estimate of the rate of TFR. The 95% CI is indicated by dashed lines.
Rate of TFR in all 40 patients. Actuarial estimate of the rate of TFR. The 95% CI is indicated by dashed lines.
Five of the 18 patients in stable TFR had detectable BCR-ABL in the blood on one (n = 4) or two occasions (n = 1), yet have not relapsed (follow-up time: 2, 15, 27, 35, or 49 months). In all of these patients, BCR-ABL was detected at a level below the threshold for MMR. These findings highlight the importance of confirming molecular relapse on two consecutive tests.
Seven late relapses occurred between 6 and 27 months (median, 14.5 months) after stopping IM. The time from detectable BCR-ABL to resumption of IM was longer in these patients (median, 89 vs 50 days), partly because of the less frequent qRT-PCR monitoring in the second year of the study and partly because of physician or patient choice to delay resumption of treatment. Four of these 7 patients showed no increase in BCR-ABL before IM was recommenced, and none lost MMR (supplementary Figure 1). The fold-rise in BCR-ABL was calculated as the increase from the detection limit of the last negative sample to the BCR-ABL value at the time of recommencing IM treatment (for example, if the detection limit of the negative sample was 0.001% [MR4.0], then the fold-rise was calculated from a baseline of 0.001%, the actual value being this level or lower).17 The median fold-rise for those patients who relapsed before 6 months was 91.5. In 13 of 15 patients, there was a ≥10-fold rise. Conversely, in the 7 later relapses there was a median 3.3-fold rise in BCR-ABL, with only 2 of 7 patients having ≥10-fold rise. The BCR-ABL doubling times were calculated as previously described.17 The doubling time could not be calculated for one of the 7 late relapses because the BCR-ABL fold-rise was negative (ie, the patient had spontaneously regained UMRD at the time of recommencing IM). For the 15 early relapses, the median BCR-ABL doubling time was 12.8 days (range, 8.4 to 31.9 days) vs a doubling time of 72.2 days (range, 43 to 485 days) for the 6 evaluable late relapses.
Response to retreatment
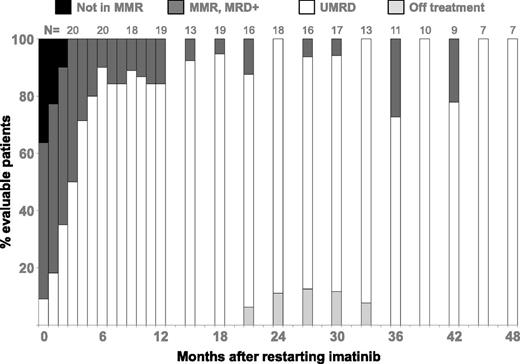
All patients who met the study definition of molecular relapse resumed IM treatment. All of the 8 patients who lost MMR regained this response after a median interval of 2 months (range, 1 to 3 months; Figure 2). All of the 22 patients who relapsed had at least one UMRD sample after restarting IM. The median time to the first UMRD sample was 3 months (range, 0 to 17 months). Nineteen of 22 patients had at least 24 months of follow-up after commencing retreatment: 16 had regained stable UMRD, 1 had intermittent UMRD, and 2 had again stopped IM.
Response to retreatment with IM after molecular relapse. Four years of follow-up after molecular relapse is shown. The number of evaluable patients at each time point is shown at the top of the column. Because of the smaller number of observations at later time points, the data after 12 months are grouped to show the 3 months prior to the stated time point (ie, results for 15 months are all results for the interval 13-15 months). If a patient had two qRT-PCR results in an interval, the patient is scored only once with the highest recorded BCR-ABL level.
Response to retreatment with IM after molecular relapse. Four years of follow-up after molecular relapse is shown. The number of evaluable patients at each time point is shown at the top of the column. Because of the smaller number of observations at later time points, the data after 12 months are grouped to show the 3 months prior to the stated time point (ie, results for 15 months are all results for the interval 13-15 months). If a patient had two qRT-PCR results in an interval, the patient is scored only once with the highest recorded BCR-ABL level.
The two patients who stopped IM treatment a second time both did so after regaining stable UMRD. One of these patients stopped after 16 months of UMRD and relapsed 11 months later. The second patient stopped after 18 months of UMRD and relapsed 2 months later.
Predictors of relapse
We analyzed multiple clinical parameters as potential predictors of relapse risk, including age, sex, Sokal score at diagnosis, IM dose and duration, time to achieve molecular response (MMR, MR4.0, and UMRD) and UMRD duration. Rates of TFR for categorical variables and for continuous variables dichotomized about the median are shown in Table 2. ROC curve analysis was performed for all continuous variables. Significant interaction with relapse risk was observed only for duration of IFN treatment and time to achieve UMRD after switching from IFN to IM treatment. Three (75%) of 4 Sokal high-risk patients relapsed vs 45% of low-risk patients (log-rank P > .05).
Clinical variables and probability of TFR 3 years after stopping IM
| . | N . | Rate of TFR . |
|---|---|---|
| Categorical variables | ||
| Sex | ||
| Male | 19 | 0.526 |
| Female | 21 | 0.367 |
| Sokal score | ||
| High | 4 | 0.250 |
| Intermediate | 16 | 0.365 |
| Low | 20 | 0.511 |
| Prior IFN treatment | ||
| Yes | 21 | 0.519 |
| No | 19 | 0.337 |
| Continuous variables | (below/above median) | |
| Age (y) | 40 | 0.550/0.328 |
| Duration of IM (mo) | 40 | 0.429/0.450 |
| Cumulative IM dose (g) | 40 | 0.400/0.458 |
| Duration of stable UMRD (mo) | 37 | 0.500/0.337 |
| . | N . | Rate of TFR . |
|---|---|---|
| Categorical variables | ||
| Sex | ||
| Male | 19 | 0.526 |
| Female | 21 | 0.367 |
| Sokal score | ||
| High | 4 | 0.250 |
| Intermediate | 16 | 0.365 |
| Low | 20 | 0.511 |
| Prior IFN treatment | ||
| Yes | 21 | 0.519 |
| No | 19 | 0.337 |
| Continuous variables | (below/above median) | |
| Age (y) | 40 | 0.550/0.328 |
| Duration of IM (mo) | 40 | 0.429/0.450 |
| Cumulative IM dose (g) | 40 | 0.400/0.458 |
| Duration of stable UMRD (mo) | 37 | 0.500/0.337 |
All comparisons P > .05.
IFN dose, duration, and response
Fourteen of the 16 patients with qRT-PCR data available at the time of switching from IFN to IM had achieved a partial cytogenetic response (ie, BCR-ABL <10%; Table 3). The rate of TFR was higher in the IFN-IM cohort (51.9%) than in the IM-only cohort (33.7%; P > .05). Following the ROC curve analysis, the IFN-IM cohort was divided according to duration of IFN treatment (<12 months [n = 5] or >12 months [n = 16]). A longer duration of IFN treatment was associated with a higher rate of TFR (62% vs 20%; P = .05). For patients who achieved UMRD within 9 months of switching from IFN to IM treatment (n = 11), there was a higher rate of TFR (82% vs 0%; P < .001) than in those with later UMRD (n = 6). In the IFN-IM cohort the depth of molecular response at the time of starting IM was not associated with relapse risk (Table 3), arguing that early achievement of UMRD after switching from IFN to IM is not simply a surrogate for the depth of response to IFN.
Molecular response to IFN or IM treatment and probability of TFR 3 years after stopping IM
| . | . | . | Proportion of patients in TFR . | . | |
|---|---|---|---|---|---|
| Median . | Range . | <Median . | >Median . | P . | |
| IFN-IM cohort | |||||
| BCR-ABL% at IM start (n = 16) | 0.76 | 0-86 | 0.600 | 0.500 | .48 |
| Days to MR4.0 (n = 18) | 258 | 78-1199 | 0.667 | 0.444 | .27 |
| Days to first UMRD (n = 17) | 244 | 89-1841 | 0.750 | 0.333 | .04 |
| IM-only cohort | |||||
| Days to MMR (n = 16) | 177 | 80-495 | 0.469 | 0.375 | .46 |
| Days to MR4.0 (n = 17) | 427 | 160-1209 | 0.375 | 0.444 | .95 |
| Days to first UMRD (n = 17) | 475 | 160-1608 | 0.429 | 0.400 | .53 |
| . | . | . | Proportion of patients in TFR . | . | |
|---|---|---|---|---|---|
| Median . | Range . | <Median . | >Median . | P . | |
| IFN-IM cohort | |||||
| BCR-ABL% at IM start (n = 16) | 0.76 | 0-86 | 0.600 | 0.500 | .48 |
| Days to MR4.0 (n = 18) | 258 | 78-1199 | 0.667 | 0.444 | .27 |
| Days to first UMRD (n = 17) | 244 | 89-1841 | 0.750 | 0.333 | .04 |
| IM-only cohort | |||||
| Days to MMR (n = 16) | 177 | 80-495 | 0.469 | 0.375 | .46 |
| Days to MR4.0 (n = 17) | 427 | 160-1209 | 0.375 | 0.444 | .95 |
| Days to first UMRD (n = 17) | 475 | 160-1608 | 0.429 | 0.400 | .53 |
IM dose, duration, and response
The prescribed daily dose of IM at the time of study entry ranged from 150 to 800 mg. Most patients were given doses of 400 mg (n = 27) or 600 mg (n = 9) daily. Details of dosing during the entire period of treatment were not available, but we estimated the total dose by assuming a constant dose over the treatment period. The median cumulative IM dose (days of treatment multiplied by daily dose) was 916 g. There was no difference in relapse risk according to duration of IM treatment, cumulative IM dose, or duration of stable UMRD (Table 2). We postulated that patients with a rapid molecular response to IM would be less likely to relapse. Among the IM-only patients, there were no differences in the risk of relapse according to the time taken to achieve MMR, MR4.0 or UMRD (Table 3).
MRD analysis by BM qRT-PCR
BM aspiration was performed at study entry and then every 3 months for up to 2 years in patients remaining in TFR. qRT-PCR was performed on BM samples at these time points for the purpose of comparing sensitivity with the PB qRT-PCR. For this analysis, there were 150 paired blood and BM qRT-PCR results. The mean number of copies of BCR was similar at >900 000 (equivalent to an average lower limit of detection of 0.0016% or MR4.8) in both PB and BM (paired Student t test P = .38).
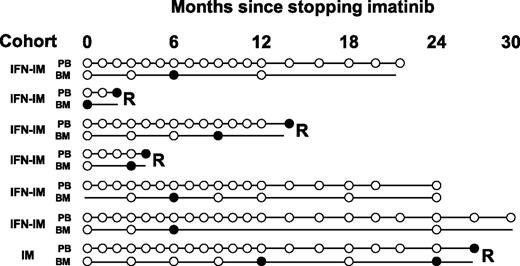
Of the 22 patients who relapsed, 4 had detectable BCR-ABL in the BM on a single occasion before BCR-ABL was detected in the PB(lead times, 1 to 15 months; Figure 3). The 2 patients who relapsed within 2 months of having detectable BCR-ABL in the BM had positive BM qRT-PCR at study entry or at 3 months. Conversely, 3 (17%) of the 18 patients in stable TFR had detectable BCR-ABL in the BM on one occasion (all at 6 months), yet remained in TFR (Figure 3). Hence, only 4 (57%) of 7 patients with an isolated positive BM qRT-PCR developed subsequent molecular relapse, a proportion that is similar to the pretest probability of relapse.
BM qRT-PCR is not clinically useful as a predictor of molecular relapse. Seven patients had detectable BCR-ABL mRNA in BM on at least one occasion while PB monitoring indicated stable UMRD. Samples with no detectable BCR-ABL are shown as open circles, and positive samples are shown as solid circles. Molecular relapse is denoted by “R” at the end of the timeline. The BM qRT-PCR indicated imminent molecular relapse in only 2 patients.
BM qRT-PCR is not clinically useful as a predictor of molecular relapse. Seven patients had detectable BCR-ABL mRNA in BM on at least one occasion while PB monitoring indicated stable UMRD. Samples with no detectable BCR-ABL are shown as open circles, and positive samples are shown as solid circles. Molecular relapse is denoted by “R” at the end of the timeline. The BM qRT-PCR indicated imminent molecular relapse in only 2 patients.
MRD analysis by DNA PCR
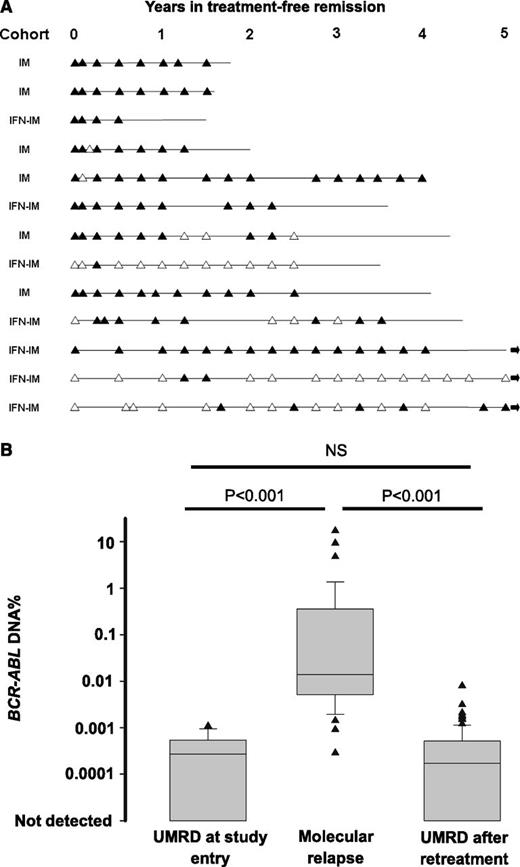
Genomic DNA PCR for BCR-ABL was performed on PB samples from 26 patients. Thirteen of these patients relapsed and 13 remained in TFR at last follow-up. DNA PCR could not be performed in the remaining 14 patients because no suitable diagnostic sample was available for break-point detection. Our earlier report in a smaller number of patients showed that the detection of BCR-ABL DNA at study entry had a low positive predictive value for subsequent molecular relapse. Updated results from 25 patients (one baseline sample was missing) confirm a positive predictive value of 53% and a negative predictive value of 67%. Notably, all of the 13 patients in stable TFR had detectable BCR-ABL DNA on at least one occasion during follow-up (Figure 4A), so the detection of BCR-ABL DNA lacked prognostic utility.
BCR-ABL DNA evidence of residual disease in patients with UMRD. (A) Results of BCR-ABL DNA monitoring for 13 patients in TFR. Solid triangles indicate that BCR-ABL DNA was detected; open triangles indicate that BCR-ABL DNA was not detected. The horizontal line shows the current period of follow-up for each patient. An arrow at the end of the line indicates that the patient remains in stable TFR with follow-up of more than 5 years. (B) The average level of BCR-ABL DNA for 13 patients who met the study definition of molecular relapse showing all results from patients with UMRD at study entry, all results at molecular relapse, and all results after regaining UMRD on IM re-treatment. Quantitative results are expressed relative to the individual patient’s baseline diagnostic sample. NS, not significant.
BCR-ABL DNA evidence of residual disease in patients with UMRD. (A) Results of BCR-ABL DNA monitoring for 13 patients in TFR. Solid triangles indicate that BCR-ABL DNA was detected; open triangles indicate that BCR-ABL DNA was not detected. The horizontal line shows the current period of follow-up for each patient. An arrow at the end of the line indicates that the patient remains in stable TFR with follow-up of more than 5 years. (B) The average level of BCR-ABL DNA for 13 patients who met the study definition of molecular relapse showing all results from patients with UMRD at study entry, all results at molecular relapse, and all results after regaining UMRD on IM re-treatment. Quantitative results are expressed relative to the individual patient’s baseline diagnostic sample. NS, not significant.
All 13 patients in the DNA PCR subgroup who relapsed had detectable BCR-ABL DNA in the PB at or before the time of molecular relapse (as defined by qRT-PCR). The DNA PCR assay is semiquantitative at low levels of residual disease with low precision, as is common in an assay operating near the limit of detection. We compared the level of BCR-ABL DNA for all 13 patients who experienced molecular relapse at study entry (baseline or 1 month after stopping, with UMRD), at molecular relapse (samples with detectable BCR-ABL mRNA), and after regaining UMRD on IM retreatment for at least 6 months. For the purposes of this analysis, samples with no detectable BCR-ABL DNA were assigned a value of 0.00001%. In patients who relapsed, BCR-ABL DNA increased significantly and then decreased following retreatment with IM (Figure 4B). The level of BCR-ABL DNA after regaining stable UMRD was similar to that at study entry and was stable, with most values in a range of approximately 20-fold variation (Figure 4B).
Discussion
The finding that approximately 40% of chronic-phase CML patients with stable UMRD being given IM can stop treatment and remain in TFR is clinically important and reproducible. Two independent prospective multicenter studies—this study in Australia and the STIM study in France—have shown almost identical rates of TFR in patients with similar enrollment criteria and similar qRT-PCR sensitivity. All patients with molecular relapse remained sensitive to IM treatment. No patient developed resistant disease or hematologic progression.
We recently reviewed the qRT-PCR results of 423 patients treated with first-line IM and monitored in the Adelaide laboratory. After 8 years of IM treatment the cumulative incidence of stable UMRD for 24 months (the key TWISTER eligibility criterion) was 36.5%.18 In the IM-only cohort of TWISTER, the rate of TFR was 33.7%. Therefore, we estimate that approximately 12% of all first-line IM -treated CML patients may eventually achieve TFR.
Although there is considerable interest in TKI cessation, a lack of standard definitions of molecular response and sensitivity of molecular testing has hampered the process.19 We have avoided the widely used term “complete molecular response” since the completeness of a molecular response is wholly dependent on the sensitivity of the PCR assay in question. This is clearly demonstrated by the results of more sensitive PCR testing and by the occurrence of molecular relapse in 50% to 60% of patients with UMRD who stop treatment.6,15,20,21 UMRD is a more precise description of the level of response achieved in these patients.
It should be emphasized that the term “relapse” in this study refers to a level of measurable MRD that is associated with little or no risk of clinical adverse outcomes. Examining the timing and kinetics of relapse, we saw two distinct patterns: those in the first few months were consistently associated with a logarithmic increase in BCR-ABL, with a doubling time similar to that reported in other studies,6,17,22,23 whereas most of the later relapses were associated with much longer doubling times or, in some cases, with no significant increase in BCR-ABL.
The most appropriate definition of molecular relapse for clinical purposes remains to be established. In retrospect, it may be that our definition of relapse was too broad, because it resulted in some patients with UMRD recommencing IM therapy. However, since our patients resumed IM, we do not know whether loss of MMR would inevitably have followed. Nevertheless, the vast majority of patients who met the TWISTER definition of molecular relapse before 6 months had substantial increases in BCR-ABL and short doubling times. It seems likely that these patients would rapidly lose MMR, so in this situation, the criterion of two consecutive positive tests, even below the level of MMR, seems appropriate. Relapses from 6 months onward showed slower kinetics with less certainty that progressive loss of response would follow. For this situation, loss of MMR may be a more robust indication to resume TKI therapy.
MRD analysis in acute leukemia has shown higher sensitivity in the BM than in the PB,24,25 so we tested whether BM qRT-PCR might be more sensitive than PB qRT-PCR. Importantly, we found no evidence that ongoing BM aspiration every 3 months for qRT-PCR provides any advantage over PB monitoring. The isolated detection of low-level BCR-ABL in either PB or BM had poor predictive value for relapse. On the basis of this analysis, we would not recommend regular BM qRT-PCR in addition to PB qRT-PCR as a monitoring regimen in future cessation studies.
We examined a number of clinical parameters that might predict for relapse risk. This study was not adequately powered to show statistically significant differences between multiple subgroups. Nevertheless, some of the differences that we found were consistent with those of the larger STIM study, including a higher risk of relapse in patients with higher Sokal scores. In a Korean survey of 14 CML patients who stopped IM therapy, all 7 patients with a high-risk Sokal score experienced molecular relapse.11 In our study, there were more Sokal low-risk patients in the IFN-IM cohort, and this may be a confounding factor when considering the effect of prior IFN, since patients with more favorable disease biology may be more likely to persist with IFN treatment.
The STIM study found a lower risk of relapse in IFN-IM patients,6 as did our study. In a subgroup analysis of our IFN-IM cohort, a longer duration of IFN treatment (≥12 months) was associated with a lower risk of relapse. Whether this reflects synergistic effects of sequential IFN and IM treatment or whether this association is confounded by the selection of a subgroup of patients with favorable disease biology (IFN responsive) cannot be determined. A Japanese survey of IM withdrawal also reported a lower risk of relapse with prior IFN treatment.7 TFR in CML has previously been reported in patients treated with IFN alone.26 We also found that patients who achieved UMRD early (within 9 months) after switching from IFN to IM therapy were much less likely to relapse. This effect was not attributable simply to the level of MRD at the time of switching to IM, since the average level of MRD was no different between early and late responders. In contrast, no benefit of early UMRD was seen in the IM-only cohort.
The low positive predictive value of a single isolated positive qRT-PCR for molecular relapse might be due to false-positive results, since with very frequent testing, the likelihood of a rare false-positive test increases. For this reason, we developed patient-specific genomic BCR-ABL DNA PCR for MRD analysis.15 The diversity of genomic break points ensures that each patient’s fusion gene is a unique marker of that CML clone. BCR-ABL DNA PCR enabled earlier detection of impending molecular relapse, but the lead time was too short to be clinically useful.15 For patients who relapsed and resumed IM treatment, the level of residual disease after treatment was similar to that at study entry, arguing that there is no significant loss of disease control after a carefully monitored trial of IM withdrawal.
The detection of BCR-ABL DNA at study entry was not helpful in predicting relapse risk, but it gave important insights into the biology of TFR. TFR can be maintained despite the persistence of the original leukemic clone, and the level of residual disease may remain stable over several years of follow-up without treatment. A similar phenomenon has been reported using qRT-PCR monitoring of patients in remission late after allogeneic stem cell transplantation.27-29
The presence of stable BCR-ABL DNA evidence of MRD off treatment was unexpected and remains unexplained. We speculate that this might be a result of ongoing immunologic suppression of the CML clone. There is significant circumstantial evidence to support this hypothesis, including the curative potential of donor lymphocyte infusion after relapse following allogeneic stem cell transplantation.30 Since IFN has pleiotropic immune effects in CML,31-33 the possible association of prolonged IFN treatment with TFR might also be relevant, acknowledging that duration of IFN treatment is related to efficacy and might reflect favorable disease biology.
Alternative hypotheses have been proposed that might account for the observation of sustained TFR, including clonal exhaustion and subclonal selection.34 In the clonal exhaustion model, stochastic commitment of true CML stem cells to differentiation and proliferation leaves no stem cells to perpetuate the disease.35 Since committed CML progeny survive only for a finite period, our observation of stable levels of CML for several years would not support this hypothesis. The type of cells in which BCR-ABL DNA is detected in TFR is not known, and therefore we cannot exclude the possibility that apparent MRD is actually due to the presence of a long-lived cell, such as a T lymphocyte,36 that is derived from the CML clone but cannot recapitulate leukemia. In the subclonal selection model, durable TFR is due to the persistence of leukemic subclones (selected during IM treatment) with diminished proliferative capacity.37 This model could explain the late relapses that we observed. However, the stable level of BCR-ABL DNA in TFR, persisting for more than 5 years, argues against the presence of even a slowly proliferating subclone in those patients with the longest follow-up.
In this study, the high standard of qRT-PCR in a central laboratory, the frequent monitoring, and the adherence to strict criteria for molecular relapse were key factors to ensure patient safety. Experience with TKI cessation outside clinical trials is limited, and less stringent monitoring might result in delayed resumption of treatment of patients who relapse. While acknowledging these potential dangers, on the basis of this study and other published studies, we believe that a carefully monitored trial of IM withdrawal can be undertaken safely in selected CML patients. Approximately 40% of CML patients with stable UMRD taking IM for at least 2 years are likely to remain in a prolonged TFR after treatment is stopped.
The online version of this article contains a data supplement.
There is an Inside Blood commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank the patients who took part in this study, the clinical trials staff at the various hospitals, and the current and former staff of the Australasian Leukaemia & Lymphoma Group Trial Centre who have contributed to the study: John Reynolds, Rachel Koelmeyer, and Michael Kornhauser.
This work was supported by research funding from Novartis Pharmaceuticals (to the ALLG), a Leukaemia Foundation of Australia scholarship (D.M.R.), and a National Health and Medical Research Council Practitioner Fellowship (T.P.H.).
Authorship
Contribution: D.M.R., S.B., and T.P.H. designed and performed research, analyzed the data, and wrote the manuscript; J.F.S., A.P.S., J.V.M., D.L.W., and A.P.G. designed and performed research and wrote the manuscript; C.A., D.T.Y., P.D., J.M.G., R.J.F., and A.K.M. performed research and reviewed the manuscript; C.S. provided essential logistical support and reviewed the manuscript.
Conflict-of-interest disclosure: D.M.R., S.B., D.L.W., and T.P.H. have received research funding and honoraria from Novartis Pharmaceuticals. J.F.S., A.P.S., C.A., D.T.Y., and A.K.M. have received honoraria from Novartis Pharmaceuticals. C.S. is an employee of Novartis Pharmaceuticals. The remaining authors declare no competing financial interests.
Correspondence: David M. Ross, Haematology Department, Room 6D110, Flinders Medical Centre, Bedford Park, SA 5042, Australia; e-mail:david.ross@health.sa.gov.au.