To the editor:
Inherited thrombocytopenias form a heterogenous group of diseases characterized by decreased platelet count and increased risk of bleeding. Mutations in at least 17 genes have been associated with autosomal-recessive, autosomal-dominant, and X-linked forms of the disease (recently reviewed by Balduini and Savoia1 ). Autosomal-dominant nonsyndromic thrombocytopenia-2 (THC2; MIM 188000) is characterized by decreased platelet count, mild propensity to bleeding, normal platelet function, normal numbers of megakaryocytes, and normal maturation stages, suggesting defective platelet production or release.2 Morphologically, platelets and megakaryocytes appear normal under light microscopy, but a recent study suggests the presence of particulate cytoplasmic structures in ANKRD26 platelets and megakaryocytes when examined under electron microscopy.3 Genetic studies have identified mutations of the 5′ untranslated region (UTR) of the ANKRD26 gene as the underlying cause of the phenotype.4 Since the initial report, additional mutations have been described in the same 22-nucleotide stretch of the 5′ UTR in a total of 21 pedigrees that are postulated to cause THC2 by upregulation of ANKRD26 expression.2,4
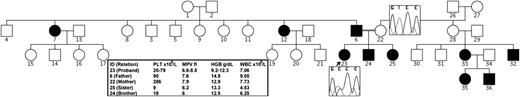
We carried out a whole-exome sequencing experiment on 2 individuals, an affected father-daughter pair, from a 4-generation Saudi Arabian family with autosomal-dominant nonsyndromic thrombocytopenia (Figure 1). The proband, a 4-year-old girl, presented with a history of bruising. Initial investigations revealed mild thrombocytopenia and iron deficiency anemia requiring only 3 transfusions since birth. Laboratory investigations revealed the following: hemoglobin, 12.2 g/dL; white blood cells, 11.08 × 109/L; neutrophil count, 5.7 × 109/L; platelet count, 26 × 109/L (mean platelet volume, 7.8); hypochromic and microcytic red blood cells; normal prothrombin time and partial thromboplastin time; and low serum ferritin. A bone marrow examination showed normal cellularity with megakaryocytes, a few of which were hypoglobulated. The bone marrow iron store was critically low. Additional laboratory values are displayed in Figure 1.
Family pedigree. Black circles and squares indicate affected individuals/D158G heterozygotes. Chromatogram traces are shown for the proband and her wild-type mother. HGB, hemoglobin; MPV, mean platelet volume; PLT, platelet; WBC, white blood cell.
Family pedigree. Black circles and squares indicate affected individuals/D158G heterozygotes. Chromatogram traces are shown for the proband and her wild-type mother. HGB, hemoglobin; MPV, mean platelet volume; PLT, platelet; WBC, white blood cell.
The whole-exome sequencing generated a total of 317 950 single-nucleotide variations and 28 358 indels in the daughter and 277 830 single-nucleotide variations and 24 797 indels in the father. Of those, 178 variants in 175 genes were shared between the 2 affected individuals and predicted to have a deleterious effect on protein function. After excluding variants present in public databases at a frequency of >1%, 122 variants in 118 genes remained.
Those variants included a missense mutation, [c.A473G:p.D158G], in ANKRD26 shared by both the affected father and daughter and absent from a total of 8228 control subjects. Sanger sequencing was used to validate the mutation in the proband and confirm transmission of the mutation in the extended pedigree. The aspartic acid residue at position 158 is not highly conserved through evolution, but we have found no evidence of substitutions to a glycine among 40 species,5 which would not preclude a functional effect on the protein.
Phenotypically, carriers of 5′ UTR mutations and the D158G missense mutation appeared very similar. Bleeding was mild, platelet morphology was normal under light microscopy, and megakaryocytes were normal. Noncoding mutations of ANKRD26 have recently been reported to be causal of THC2 after false starts with the MASTL and ACBD5 genes. Our study adds support to the role of ANKRD26 in THC2 and suggests missense mutations may also paly a role in the pathogenesis of THC2.
Authorship
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Hakon Hakonarson, The Center for Applied Genomics, The Children’s Hospital of Philadelphia, 3615 Civic Center Blvd, Office 1016J, Philadelphia, PA 19104-4318; e-mail: hakonarson@email.chop.edu; and Patrick Sleiman, The Center for Applied Genomics, The Children’s Hospital of Philadelphia, 3615 Civic Center Blvd, Office 1016J, Philadelphia, PA 19104-4318; e-mail: sleimanp@email.chop.edu.