To the editor:
We read with great interest the article by Liapis and Colleagues1 that was published in Blood on May 7, 2013 (online ahead of print). The authors investigated the tumor, microenvironment, and viral components in AIDS-related diffuse large B-cell lymphoma (DLBCL). The results of the study showed that AIDS-related DLBCL is highly angiogenic, with markedly higher blood-vessel density than sporadic DLBCL cases. Importantly, the investigation also highlighted the role of Epstein-Barr virus (EBV) in angiogenesis.1 In a previous work,2 we used gene expression profile (GEP) analysis (∼12 000 genes) to further define the phenotype of AIDS-related non-Hodgkin lymphoma (AIDS-NHL).2 The AIDS-NHL cases selected for the study included several subtypes displaying distinct histologic appearance. Indeed, the spectrum of AIDS-NHL ranged from DLBCL of the centroblastic or immunoblastic type to primary effusion lymphoma and Burkitt lymphoma. In agreement with their distinct morphologic appearance, the results indicated that EBV-positive AIDS-DLBCL of the immunoblastic type (AIDS-DLBCL-IB) represented a separate entity relative to AIDS-NHL. Among the various subtypes of AIDS-NHL, EBV-positive AIDS-DLBCL-IB seemed to be more similar to primary effusion lymphoma.2
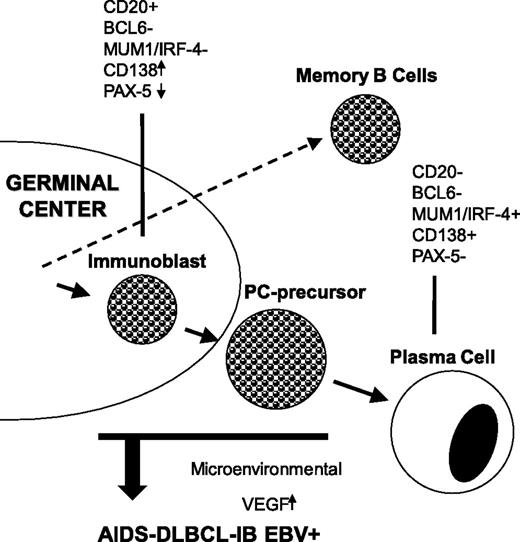
Since an additional aim of the original work2 was to investigate the relationship of AIDS-NHL to normal B cells and to AIDS-unrelated NHL, we would like to raise 2 more questions about EBV-positive AIDS-DLBCL-IB: (1) what is the relationship with the supposed lymphoma counterpart in immunocompetent hosts?, and (2) where do tumor cells derive from? To determine the relationship with the lymphoma counterpart in the immunocompetent hosts, we compared GEPs by unsupervised and supervised analyses. The results of the comparative analysis revealed that AIDS/Burkitt lymphoma and AIDS-DLBCL of the centroblastic type, but not EBV-positive AIDS-DLBCL-IB, were indistinguishable from their counterparts in immunocompetent hosts. To define the cellular origin, we compared the GEPs of the individual AIDS-NHL cases with the specific gene expression signatures of normal B-cell subsets. We included in the comparative analysis EBV-immortalized B-cell lines as representative of immunoblasts and multiple myeloma cell lines as representative of the terminally differentiated plasma cell stage. We found relatedness of the GEP of the EBV-positive AIDS-DLBCL-IB cases to the multiple myeloma cell lines. In summary, EBV-positive AIDS-DLBCL-IB represented a distinct entity when compared with the other AIDS-NHL subtypes and its supposed counterpart in immunocompetent hosts. Moreover, by GEP analysis, EBV-positive AIDS-DLBCL-IB displayed a phenotype related to plasma cells (Figure 1).3 This finding was not surprising, since it is known that EBV infection in AIDS-DLBCL is consistently linked to plasmacytoid/plasmablastic differentiation.4
EBV-positive AIDS-related DLBCLs of the immunoblastic type display a phenotype related to plasma cells. The tumor cells display immunoblast-associated antigens together with plasma-cell–associated markers. The figure also shows the putative role of EBV in the microenvironmental angiogenesis by inducing vascular endothelial growth factor (VEGF) upregulation.
EBV-positive AIDS-related DLBCLs of the immunoblastic type display a phenotype related to plasma cells. The tumor cells display immunoblast-associated antigens together with plasma-cell–associated markers. The figure also shows the putative role of EBV in the microenvironmental angiogenesis by inducing vascular endothelial growth factor (VEGF) upregulation.
Although latency programs predominate in EBV-driven tumors, lytic EBV replication may also be of pathogenic relevance, at least in the early phases of cell transformation.5,6 This finding is particularly relevant for AIDS-related lymphomagenesis, since the underlying impairment of immune responses may favor uncontrolled activation of EBV lytic replication in latently infected B lymphocytes. Importantly, regarding tumor microenvironment, EBV infection is implicated in angiogenic mechanisms.5-7 In conclusion, the results reported by Liapis and colleagues1 are consistent with genetic and virological data suggesting that in AIDS-DLBCL, neovascularization is linked to EBV status of tumor and to immunoblastic features.
Authorship
Contribution: A.C. designed the paper; and A.C. and A.G. wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Antonino Carbone, Centro di Riferimento Oncologico Aviano, Istituto Nazionale Tumori, IRCCS, Via F. Gallini 2, 33081 Aviano, Italy; e-mail: acarbone@cro.it.