Key Points
Fev is required for endothelium-based HSC emergence.
Fev directly regulates ERK signaling to regulate HSC development cell-autonomously.
Abstract
Reprogramming of somatic cells to desired cell types holds great promise in regenerative medicine. However, production of transplantable hematopoietic stem cells (HSCs) in vitro by defined factors has not yet been achieved. Therefore, it is critical to fully understand the molecular mechanisms of HSC development in vivo. Here, we show that Fev, an ETS transcription factor, is a pivotal regulator of HSC development in vertebrates. In fev-deficient zebrafish embryos, the first definitive HSC population was compromised and fewer T cells were found in the thymus. Genetic and chemical analyses support a mechanism whereby Fev regulates HSC through direct regulation of ERK signaling. Blastula transplant assay demonstrates that Fev regulation of HSC development is cell autonomous. Experiments performed with purified cord blood show that fev is expressed and functions in primitive HSCs in humans, indicating its conserved role in higher vertebrates. Our data indicate that Fev-ERK signaling is essential for hemogenic endothelium-based HSC development.
Introduction
Hematopoietic stem cells (HSCs) can give rise to all blood lineages, including erythroid (erythrocyte), myeloid (macrophage, neutrophil, monocyte), and lymphoid (B cell and T cell). HSC transplantation has been successfully applied in the clinic to treat hematological diseases such as leukemia. However, the shortage of sources (mainly compatible bone marrow) has restricted its application worldwide. Production of transplantable HSCs in vitro holds great promise for solving this issue but has not been successfully achieved from embryonic or induced pluripotent stem cells or by defined factors. Therefore, a full understanding of molecular mechanisms of HSC development in vivo is critical for regenerative medicine application of HSCs expanded ex vivo or in vitro. During embryogenesis, the first HSCs are believed to be derived from the ventral wall of the dorsal aorta (ie, hemogenic endothelium) through endothelial-hematopoietic-transition (EHT) in the embryos of zebrafish, Xenopus, chick, and mammals.1-8 However, the underlying molecular mechanism of hemogenic endothelium-derived HSC differentiation remains to be determined.
Several transcription factors have been shown to be essential for HSC development.9 Runx1 is a known master regulator of HSC specification that is expressed in the ventral wall of the dorsal aorta, the underlying mesenchyme, and intra-aortic hematopoietic clusters in mice.10,11 Loss-of-Runx1 in mice completely abolished HSC formation in the dorsal aorta10 ; however, it did not affect the formation of the dorsal aorta from which HSCs are derived. cmyb is expressed in long-term HSCs and cmyb-null mice failed to develop adult hematopoiesis in the fetal liver, but the commitment to definitive hematopoiesis can occur in the absence of cMyb.12 Therefore, identification of upstream regulators of Runx1 and cMyb is essential for understanding the origin of HSC development from hemogenic endothelium. Several ETS transcription factors act individually or combinatorially to regulate dorsal aorta13,14 and/or HSC development and functions.15-17 In Xenopus embryos, we previously showed that the ETS factor Tel1/ETV6 specifies the first HSCs in the dorsal aorta by regulating the expression of vegfa in both the lateral plate mesoderm and the somites, suggesting cell-autonomous and non-cell–autonomous roles for Tel1 in HSC development.16 In zebrafish, we identified a new ETS gene, fev (also known as pet1 in mammals), which belongs to the same subgroup as fli1 and erg.18 Fev was initially reported to be expressed in serotonergic neurons in vertebrates and in mice lacking Pet-1 the majority of serotonin-producing (5-hydroxytryptamine) neurons fail to differentiate and the level of 5-hydroxytryptamine is decreased by 70% to 80%.19-21 However, whether fev plays a role during HSC development remains unknown.
Here, we demonstrate that Fev, an ETS transcription factor, is a pivotal regulator of HSC development in zebrafish and humans. Fev acts upstream of ERK signaling to regulate HSC specification and function, which is distinct from its well-known role in serotonergic neuron development in vertebrates.
Methods
Zebrafish husbandry
Zebrafish strains including AB, fli1a:GFP (generously provided by S. Wilson), fli1a-nuclear-GFP (fli1a:nGFP) and cmyb:GFP (generously provided by A. Meng), and kdrl:mCherry (from B. Zhang laboratory in Peking University) transgenic lines were raised and maintained at 28.5°C in system water and staged as previously described.22 This study was approved by the Ethical Review Committee in the Institute of Zoology, Chinese Academy of Sciences, China and was conducted in accordance with the Declaration of Helsinki.
Morpholinos, mRNA synthesis, and microinjection
The detailed protocols for these assays are described in supplemental Methods.
Chemical treatment
Zebrafish embryos were incubated with fish water containing LY294002 or U0126 (final concentration 10 µM) from bud stage, while embryos incubated with fish water containing 0.1% dimethylsulfoxide (vehicle alone) served as control. During the next 24 h, the embryos were periodically examined to ensure that the pharmacological effect remained constant over time. Chemicals including LY294002 and U0126 were purchased from Sigma.
Whole mount in situ hybridization
Immunofluorescence
Anti-phosphorylated ERK (Cell Signaling) staining was performed on transverse sections as previously described.24
RT-PCR and qPCR
Reverse transcription-polymerase chain reaction (RT-PCR) and quantitative PCR (qPCR) were performed as described23 and are described in supplemental Methods. Three independent experiments were carried out and in each experiment, 30 to 50 embryos were used for each sample. The PCR primers used and the expected product lengths are summarized in supplemental Table 2.
Western blot, TUNEL assay, and BrdU labeling, reporter assay, and Fev polyclonal antibody production
The detailed protocols for these assays are described in the supplemental Methods.
ChIP assay
Chromatin immunoprecipitation (ChIP) analysis was carried out with the trunk tissues of wild-type 36 hours post fertilization (hpf) embryos, and the eluted DNA (precipitated by Fev polyclonal antibody) was assayed by PCR as previously described.23,24 The primers specific to the Fev binding site within the upstream regulatory regions of erk2 were designed by searching for the conserved binding sites and primers nonspecific for these binding sites were designed and used as control primers. The primers and the expected product length are summarized in supplemental Table 3. Rabbit purified immunoglobulin G and nonspecific primers were included as negative controls.
Blastula transplantation
Tg(cmyb:GFP) embryos were injected with fevMO or control MO with dextran tetramethylrhodamine (Red, Invitrogen) at the one-cell stage. And at the shield stage, 30 to 50 cells were removed from the donor embryos’ ventral marginal region and transplanted into the stage-matched host embryos. Host embryos were then examined and analyzed by confocal microscopy at 36 hpf.
Human cord blood assays
Cord blood (CB) from anonymous healthy donors was provided from Shanghai Cord Blood Bank in accordance with local ethics procedures, and the experiments related to human CB were approved by the local ethics committee. More details are described in supplemental Methods.
Confocal microscopy
Confocal images were acquired with a Zeiss LSM 510 META confocal laser microscope, and 3D projections were generated using Zeiss LSM software (Carl Zeiss).18
Statistical analysis
For statistical analysis, Student’s unpaired 2-tailed t test was used for all comparisons.
Results
Fev is required for HSC emergence
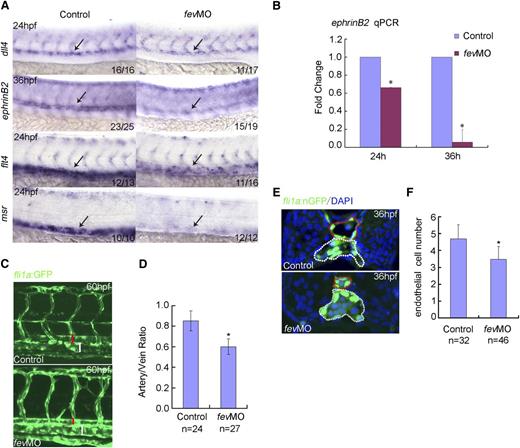
During zebrafish early embryogenesis, fev expression was initially detected bilaterally in the lateral plate mesoderm at the 5s stage and in blood/endothelial cells (ECs) at 24 hpf (Figure 1A), suggesting a role in vessel formation and hematopoiesis. To examine whether fev is required for HSC development, we used an antisense ATG MO to knock down fev expression in zebrafish. Immunoblotting analysis indicated that compared with the controls, endogenous Fev protein was markedly reduced in the morphants (Figure 1B). Whereas primitive hematopoiesis18 and vasculogenesis were relatively normal in fevMO-injected embryos (supplemental Figure 1), expression of the earliest HSC markers, runx1 and cmyb, was reduced at 24 hpf and 36 hpf (Figure 1C). qPCR analysis of runx1 and cmyb expression (Figure 1D) was consistent with WISH results, supporting the idea that the development of HSCs was disrupted significantly with fev knockdown. To further examine whether HSCs or their derivatives appear in the caudal hematopoietic tissue (CHT; the zebrafish equivalent of fetal liver in mouse) or thymus, we analyzed the expression of cmyb and the early T-cell marker, rag1, in fev morphants. cmyb expression in the CHT at 48 hpf and rag1 expression in the thymus at 4 days post fertilization (dpf) (Figure 1C) were remarkably attenuated. Furthermore, the population of cmyb:GFP labeled HSCs1 in the CHT region at 48 hpf and in the pronephros at 4 dpf and cmyb:GFP labeled T cells in the thymus at 4 dpf were much reduced in fev morphants (Figure 1E). Quantitative analysis showed that the number of GFP+ cells remarkably decreased in the CHT, thymus, and kidney in fev morphants compared with that in controls (Figure 1F).
Fev is required for HSC development. (A) Whole-mount in situ hybridization shows expression of fev in lateral plate mesoderm (arrows) at 5-somite stage and in pancreas (red arrow) and blood vessels (black arrow) at 24 hpf. The right panel shows a cross-section of 24 hpf embryo in the trunk region at the location marked by the dotted line, showing fev expression in vessels. (B) Validation of fevMO (3 ng/embryo) by western blot in control and fev morphant embryos at 24 hpf. (C) The runx1 data, expression of runx1 at 24 and 36 hpf in embryos injected with control MO or fevMO (top panels). Arrows mark runx1 expression in HSCs. The cmyb data, expression of cmyb at 36 and 48 hpf in CHT of embryos injected with control MO (middle panels). The rag1 data, expression of rag1 at 4 dpf in thymus of embryos injected with control MO or fevMO (bottom panels). Arrows indicate CHT or thymus. Anterior is to the left and dorsal is up. (D) qPCR analysis of runx1 and cmyb expression in the dissected trunk region of embryos injected with control or fevMO at 24 and 36 hpf (mean ± SD, n = 3,*P < .05). (E) Population of the GFP+ cells (hematopoietic stem/progenitor cells) in the CHT region was reduced in cmyb:GFP/kdrl:mCherry double transgenic embryos injected with fevMO compared with those injected with control MO (top panels). White arrowheads mark HSCs/HSPCs. Population of the GFP+ cells in the pronephros and T cells in the thymus at 4 dpf were much reduced in cmyb:GFP/kdrl:mCherry double transgenic embryos injected with fevMO (bottom panels). The thymus and kidney are circled in yellow and white, respectively. (F) Quantification of GFP+ cells in the CHT, thymus, and kidney between control and fev morphant embryos in E. Note that 5 control embryos and 5 fev morphants were used for counting GFP+ cells in the CHT, thymus, and kidney regions. (G) Hemogenic endothelium analysis in the AGM region using a cmyb:GFP/kdr1:mCherry double transgenic line at 36 hpf. Blue arrow, kdrl+cmyb+ cells (hemogenic ECs); white arrowhead, kdrl−cmyb+ cells (emerging HSCs). CHT, caudal hematopoietic tissue.
Fev is required for HSC development. (A) Whole-mount in situ hybridization shows expression of fev in lateral plate mesoderm (arrows) at 5-somite stage and in pancreas (red arrow) and blood vessels (black arrow) at 24 hpf. The right panel shows a cross-section of 24 hpf embryo in the trunk region at the location marked by the dotted line, showing fev expression in vessels. (B) Validation of fevMO (3 ng/embryo) by western blot in control and fev morphant embryos at 24 hpf. (C) The runx1 data, expression of runx1 at 24 and 36 hpf in embryos injected with control MO or fevMO (top panels). Arrows mark runx1 expression in HSCs. The cmyb data, expression of cmyb at 36 and 48 hpf in CHT of embryos injected with control MO (middle panels). The rag1 data, expression of rag1 at 4 dpf in thymus of embryos injected with control MO or fevMO (bottom panels). Arrows indicate CHT or thymus. Anterior is to the left and dorsal is up. (D) qPCR analysis of runx1 and cmyb expression in the dissected trunk region of embryos injected with control or fevMO at 24 and 36 hpf (mean ± SD, n = 3,*P < .05). (E) Population of the GFP+ cells (hematopoietic stem/progenitor cells) in the CHT region was reduced in cmyb:GFP/kdrl:mCherry double transgenic embryos injected with fevMO compared with those injected with control MO (top panels). White arrowheads mark HSCs/HSPCs. Population of the GFP+ cells in the pronephros and T cells in the thymus at 4 dpf were much reduced in cmyb:GFP/kdrl:mCherry double transgenic embryos injected with fevMO (bottom panels). The thymus and kidney are circled in yellow and white, respectively. (F) Quantification of GFP+ cells in the CHT, thymus, and kidney between control and fev morphant embryos in E. Note that 5 control embryos and 5 fev morphants were used for counting GFP+ cells in the CHT, thymus, and kidney regions. (G) Hemogenic endothelium analysis in the AGM region using a cmyb:GFP/kdr1:mCherry double transgenic line at 36 hpf. Blue arrow, kdrl+cmyb+ cells (hemogenic ECs); white arrowhead, kdrl−cmyb+ cells (emerging HSCs). CHT, caudal hematopoietic tissue.
The impaired HSC and T-cell development in fev morphants could be due to abnormal cell proliferation or excessive apoptosis. To test these possibilities, we employed BrdU labeling for cell proliferation and TUNEL staining for apoptosis assays (supplemental Figure 2). BrdU labeling experiments showed that decreased cell proliferation occurred in fev morphants (supplemental Figure 2A-B), suggesting that the HSC defects in fev morphants might be due to slightly reduced cell numbers. In addition, the TUNEL assay showed more apoptotic cells in fev morphants (supplemental Figure 2C) compared with the controls, indicating that the increased apoptosis might contribute to the HSC defects as well. To further explore this possibility, we used p53MO, which was previously demonstrated to prevent p53-dependent apoptosis.25,26 As shown in supplemental Figure 2C-D, p53MO knockdown can efficiently prevent ectopic apoptosis in fev morphants but failed to restore runx1 and cmyb expression in the aorta-gonad-mesonephros (AGM) region at 24 and 36 hpf, respectively. Taken together, these data suggest that the impaired HSC development in fev morphants was most likely attributed to the specification and/or proliferation defects, but not apoptosis.
To verify that the HSC defects were indeed specific to fev gene activity, we generated fev mutants by TALEN (supplemental Methods).27 We obtained 3 different indel mutants for the fev gene, and the most severe allele contained an 8-bp indel in which endogenous Fev protein levels were nearly abolished (supplemental Figure 3A-D). We examined the expression of aforementioned HSC and T-cell markers in this mutant and obtained similar results to those in fev morphants (supplemental Figure 3E-F). Western blot results further confirmed that protein levels of Fev and Runx1 were similarly reduced in fev morphants and fev TALEN mutants (supplemental Figure 3G). Taken together, these data indicate that fev is required for HSC emergence.
Hemogenic endothelium-based dorsal aorta is affected in fev-deficient embryos
The dorsal aorta functions as a prerequisite site for definitive hematopoiesis, because the first definitive HSCs are derived from the ventral wall of the dorsal aorta (ie, hemogenic endothelium) through the EHT process in fish1,2 or in the AGM in mammals.3 To monitor the hemogenic endothelium-mediated EHT process, we took advantage of a cmyb:GFP/kdr1:mCherry double transgenic line. As shown in Figure 1G, both cmyb+kdr1+ cells (ie, hemogenic endothelium) and cmyb+kdr1− cells (ie, HSCs formed through EHT) were reduced in fev morphants, suggesting hemogenic endothelium-derived HSCs were compromised. runx1 is initially expressed in a small subset of endothelium, then is restricted to hemogenic endothelium and HSCs thereafter in fish and mice.7,10 We further performed a detailed time-course assay for runx1 expression in fev morphants. It is clear that the earliest expression of runx1 in ECs at 22 hpf was not affected in fev morphants. However, from 24 hpf onwards, runx1 expression in hemogenic endothelium and the HSCs derived through EHT was increasingly compromised (supplemental Figure 4). Previous studies from us and others showed that when Shh, VEGF, Notch, or BMP signaling was disrupted, programming of artery and/or HSC was affected.7,28,29 Therefore, we next evaluated the expression of these signaling components and arterial-venous markers in fev-deficient embryos. Interestingly, there were no obvious alterations in the expression of Shh signaling, BMP signaling, or VEGF ligand (supplemental Figure 5). However, expression of dll4, an early artery marker, was modestly reduced at 24 hpf, and expression of ephrinB2, a more differentiated artery marker, dramatically decreased at 36 hpf (Figure 2A). qPCR analyzed at 24 and 36 hpf further suggests that the artery program was disrupted with fev knockdown (Figure 2B). Interestingly, expression of the venous markers, flt4, which encodes the VEGF receptor 3, and msr was also down-regulated in fev morphants (Figure 2A). To further examine the vascular defects, the vessel structure of fli1a:GFP embryos was visualized using confocal microscopy after knocking down fev. It was clearly shown that the lumen size of the dorsal aorta was significantly reduced, whereas the lumen size of the vein in the morphants was still comparable with that in the controls (Figure 2C-D). We further examined the EC numbers in the trunk vessels using fli1a:nGFP embryos. A transverse section of the trunk region showed that in control embryos, there are on average 4.7 cells within the dorsal aorta wall at 36 hpf, whereas there are about 3.4 ECs in the morphants, with statistical significance at a level of P < .05 (Figure 2E-F). These data suggest that fev deficiency disrupted the hemogenic endothelium-based dorsal aorta and that the reduced lumen size of the dorsal aorta was likely attributed to fewer ECs in the morphants.
Hemogenic endothelium-based dorsal aorta is affected in fev-deficient embryos. (A) Expression of dll4 or ephrinB2 in the dorsal aorta of embryos injected with control MO or fevMO at 24 and 36 hpf, respectively. Expression of flt4 and msr in the cardinal vein of embryos injected with control MO or fevMO at 24 hpf. Anterior is to the left and dorsal is up. (B) qPCR analysis of ephrinB2 expression at 24 and 36 hpf in the dissected trunk region of embryos injected with control MO or fevMO (mean ± SD, n = 3, *P < .05). (C) Confocal microscopy of fli1a:GFP embryos at 60 hpf injected with control MO or fevMO. Red bracket denotes dorsal aorta, white bracket denotes cardinal vein. (D) Quantification of the artery/vein ratio of the lumen size between controls and fev morphants (mean ± SD, *P < .05). (E) Transverse section of fli1a:nGFP embryos injected with control MO or fevMO at 36 hpf. Dorsal aorta and cardinal vein are denoted by dotted circles in red and white, respectively. Nuclei were shown by 4,6 diamidino-2-phenylindole staining. (F) Statistics of EC numbers of the dorsal aorta wall on transverse sections in controls embryos and fev-morphants (mean ± SD, *P < .05).
Hemogenic endothelium-based dorsal aorta is affected in fev-deficient embryos. (A) Expression of dll4 or ephrinB2 in the dorsal aorta of embryos injected with control MO or fevMO at 24 and 36 hpf, respectively. Expression of flt4 and msr in the cardinal vein of embryos injected with control MO or fevMO at 24 hpf. Anterior is to the left and dorsal is up. (B) qPCR analysis of ephrinB2 expression at 24 and 36 hpf in the dissected trunk region of embryos injected with control MO or fevMO (mean ± SD, n = 3, *P < .05). (C) Confocal microscopy of fli1a:GFP embryos at 60 hpf injected with control MO or fevMO. Red bracket denotes dorsal aorta, white bracket denotes cardinal vein. (D) Quantification of the artery/vein ratio of the lumen size between controls and fev morphants (mean ± SD, *P < .05). (E) Transverse section of fli1a:nGFP embryos injected with control MO or fevMO at 36 hpf. Dorsal aorta and cardinal vein are denoted by dotted circles in red and white, respectively. Nuclei were shown by 4,6 diamidino-2-phenylindole staining. (F) Statistics of EC numbers of the dorsal aorta wall on transverse sections in controls embryos and fev-morphants (mean ± SD, *P < .05).
Fev acts upstream of ERK signaling to establish the HSC fate
The unaltered Shh and BMP signaling in fev morphants suggests other signaling might be involved in the defects caused by fev deficiency (supplemental Figure 5). The VEGF-Notch signaling cascade is required for both artery and HSC specification in zebrafish.7,28 The down-regulation of dll4 and eprhinB2 (a target of Δ/Notch signaling) expression, together with unchanged vegfa expression in fev morphants (Figure 2A; supplemental Figure 5D), suggests that downstream components of VEGF signaling might be affected as well. The MAPK signaling proteins ERK 1/2 are major downstream effectors of signaling cascades initiated by VEGF and other growth factors, which are involved in many eukaryotic cellular processes.30 Importantly, activated ERK signaling in the vascular niche is required for maintenance and lineage-specific differentiation of HSCs.31 However, it is unclear whether ERK also plays a role in regulating HSC formation in the dorsal aorta. We therefore examined the expression of erk1 and erk2 in the fev morphants. qPCR showed that expression of erk2 but not erk1 was significantly down-regulated in fev morphants at 24 and 36 hpf, respectively (Figure 3A). Using immunofluorescence, we showed that phosphorylated ERK1/2 was reduced specifically in the dorsal aorta area but not in the somite in fev morphants (Figure 3B yellow squares). Immunoblotting analysis further demonstrated that the levels of total ERK1/2 protein and phosphorylated ERK1/2 were both reduced in fev morphants (Figure 3C). To determine whether erk2 expression is required for HSC development in zebrafish, we knocked down erk2 expression by an antisense MO at a dose optimized to avoid any early gastrulation defects.30 As shown in Figure 3D, expression of runx1 and cmyb in the AGM region was down-regulated in erk2 morphants, suggesting that erk2 expression is also required for HSCs in the AGM region after gastrulation. It is interesting to note that although Runx1 decreased in fev or erk2 morphants, the expression of Fev was unchanged in the erk2 morphants (Figure 3C), suggesting that fev drives erk2 expression. To determine whether fev expression is also regulated by MAPK signaling, we applied MEK inhibitor U0126 and AKT inhibitor LY294002, respectively, to treat wild-type embryos at the tail bud stage. The expression of fev was not affected by any of the inhibitors examined at 24 hpf (supplemental Figure 5E), indicating that Fev indeed acts upstream of VEGF-ERK signaling. Collectively, these results indicate that in zebrafish, Fev acts upstream of ERK signaling to establish the HSC program.
Fev acts upstream of ERK signaling to establish the HSC fate. (A) qPCR analysis of erk1 and erk2 expression at 24 and 36 hpf in the dissected trunk region of embryos injected with control MO or fevMO showed that erk2 but not erk1 expression in the trunk region is reduced in fev morphants (mean ± SD, n = 3,*P < .05). (B) Immunofluorescence shows pERK1/2 expression in the dorsal aorta is reduced in fev morphants (n = 5) compared with the controls (n = 5). Note that the somitic expression of pERK1/2 is not affected in fev morphants. Yellow squares indicate the dorsal aorta area. (C) Western blot showing that Runx1 protein is significantly downregulated at 36 hpf in embryos injected with fevMO or erk2MO. In addition, total ERK1/2 and pERK1/2 proteins are strongly down-regulated at 36 hpf in embryos injected with fevMO or erk2MO. Fev is downregulated in fev morphants but not altered in erk2 morphants. (D) runx1 and cmyb expression in the dorsal aorta is reduced at 36 hpf in embryos injected with erk2MO. Anterior to the left, lateral view, arrows mark the AGM region.
Fev acts upstream of ERK signaling to establish the HSC fate. (A) qPCR analysis of erk1 and erk2 expression at 24 and 36 hpf in the dissected trunk region of embryos injected with control MO or fevMO showed that erk2 but not erk1 expression in the trunk region is reduced in fev morphants (mean ± SD, n = 3,*P < .05). (B) Immunofluorescence shows pERK1/2 expression in the dorsal aorta is reduced in fev morphants (n = 5) compared with the controls (n = 5). Note that the somitic expression of pERK1/2 is not affected in fev morphants. Yellow squares indicate the dorsal aorta area. (C) Western blot showing that Runx1 protein is significantly downregulated at 36 hpf in embryos injected with fevMO or erk2MO. In addition, total ERK1/2 and pERK1/2 proteins are strongly down-regulated at 36 hpf in embryos injected with fevMO or erk2MO. Fev is downregulated in fev morphants but not altered in erk2 morphants. (D) runx1 and cmyb expression in the dorsal aorta is reduced at 36 hpf in embryos injected with erk2MO. Anterior to the left, lateral view, arrows mark the AGM region.
Fev directly regulates ERK signaling
To verify the hierarchy of this pathway, we performed epistasis experiments using HSC markers, runx1, cmyb, and the T-cell marker rag1 as phenotypic readouts. Overexpression of erk2 partially rescued the reduced expression of runx1 and cmyb in AGM and rag1 in the thymus in fev morphants (Figure 4A). qPCR and western-blot analysis agreed with the WISH results (Figure 4B-C). To determine whether Fev is located in the nucleus as a functional transcription factor, HEK293T cells overexpressing fev were subjected to cellular subfractionation analysis. As expected, Fev is only detected in the nuclear fraction extracts (supplemental Figure 6A). Interestingly, ChIP assay with the trunk region of zebrafish embryos at 36 hpf demonstrated that Fev binds to the promoter of erk2 in vivo, suggesting a direct regulation of erk2 by Fev (Figure 4D-E). Reporter assay in HEK 293 cells using the promoter constructs of erk2, which contains conserved wild-type or mutated Fev binding sites, indicated that Fev positively regulated erk2 expression in a dose-dependent manner (Figure 4F). Moreover, overexpression experiments showed that Fev can up-regulate erk2 and runx1 expression and ERK2 can up-regulate runx1 expression (supplemental Figure 6C-D). To further demonstrate that Fev regulates HSCs specifically, we performed mRNA rescue experiments. Modified mRNA, fev misRNA (with the mutated atgMO target sequence without changing amino acid coding), was generated and co-injected with fevMO into the one-cell stage embryos. Western blot showed that fev misRNA can efficiently restore Fev expression in fev morphants. Strikingly, overexpression of fev misRNA in fev morphants restored ERK, phosphorylated ERK (pERK), and Runx1 expression at 24 hpf (supplemental Figure 6B). Moreover, expression of ERK, pERK, and Runx1 was comparably decreased in fev morphants and fev TALEN mutants (supplemental Figure 3G). Taken together, these data indicate that the Fev-ERK cascade regulates HSC development.
Fev directly regulates ERK signaling. (A) erk2 mRNA can partially rescue the expression of runx1, cmyb, and rag1 in the AGM and thymus of fev morphants. The AGM region and thymus are indicated by arrows in black and red, respectively. Anterior to the left. (B) qPCR analysis of runx1 expression in control embryos, fev morphants, and rescued embryos by overexpression of erk2 mRNA (mean ± SD, n = 3, *P < .05). (C) Western-blot analysis of Runx1 expression in fev morphants and rescued embryos. (D) The Fev core binding motif and the erk2 promoter. a, The conserved core binding site of Fev. b, The schematic structure of the erk2 promoter. CF/CR are control forward and reverse primers, which are used to amplify the erk2 promoter region without the conserved Fev binding site. erk2-1F/1R represent primers used to amplify the promoter region with the conserved Fev binding site. erk2p means the erk2 promoter construct with the wild-type Fev binding site; erk2p∆ means the erk2 promoter construct with mutated Fev binding site. (E) ChIP assay showed that Fev directly binds to the erk2 promoter region. (F) Reporter assay with the promoter constructs of erk2, which contain the conserved or mutated ETS binding sites, cotransfected with pCDNA3.1(+)-fev plasmid.
Fev directly regulates ERK signaling. (A) erk2 mRNA can partially rescue the expression of runx1, cmyb, and rag1 in the AGM and thymus of fev morphants. The AGM region and thymus are indicated by arrows in black and red, respectively. Anterior to the left. (B) qPCR analysis of runx1 expression in control embryos, fev morphants, and rescued embryos by overexpression of erk2 mRNA (mean ± SD, n = 3, *P < .05). (C) Western-blot analysis of Runx1 expression in fev morphants and rescued embryos. (D) The Fev core binding motif and the erk2 promoter. a, The conserved core binding site of Fev. b, The schematic structure of the erk2 promoter. CF/CR are control forward and reverse primers, which are used to amplify the erk2 promoter region without the conserved Fev binding site. erk2-1F/1R represent primers used to amplify the promoter region with the conserved Fev binding site. erk2p means the erk2 promoter construct with the wild-type Fev binding site; erk2p∆ means the erk2 promoter construct with mutated Fev binding site. (E) ChIP assay showed that Fev directly binds to the erk2 promoter region. (F) Reporter assay with the promoter constructs of erk2, which contain the conserved or mutated ETS binding sites, cotransfected with pCDNA3.1(+)-fev plasmid.
Cell-autonomous regulation of HSC development by Fev
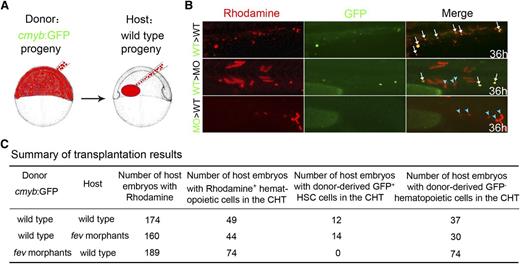
Fev expression in blood/ECs indicates that fev might act cell autonomously during HSC development (Figure 1A). To further determine whether Fev is required cell autonomously for specification of HSCs, we performed blastula transplant experiments. Donor cells labeled by rhodamine from cmyb:GFP transgenic embryos were transplanted at the blastula stage into nontransgenic recipients. A subset of rhodamine-labeled cells from the ventral marginal zone gives rise to GFP+ HSCs in the recipients. Rhodamine fluorescence indicated that 12 of 49 wild-type recipient embryos had a few GFP+ HSCs derived from control donor cells, whereas none of the donor cells from fev morphants gave rise to GFP+ HSCs in the wild-type recipient embryos (Figure 5). Conversely, 14 of 44 recipient fev morphants had GFP+ HSCs derived from control donor cells. We could not formally exclude the possibility that wild-type donor cells also repopulate the CHT niche, because we indeed noticed that there were a few cmyb:GFP−Rhodamine+ cells in this area, some of which were donor-derived GFP−hematopoietic cells (Figure 5B light blue arrowheads; Figure 5C). Taken together, these data suggest that Fev is required for HSC development cell autonomously.
Fev regulation of HSC development is cell autonomous. (A) Transplantation scheme: rhodamine-labeled donor cells at the ventral marginal zone of cmyb:GFP transgenic donor embryos were transplanted into wild-type recipients at the blastula stage. (B) HSC reconstitution in the CHT of recipient embryos at 36 hpf. Green, cmyb:GFP+ cells; red, rhodamine. White arrows, GFP+ HSCs contributed by donor cells; light blue arrowheads, donor-derived GFP− hematopoietic cells. (C) Summary of transplantation results. Note that only host embryos with donor-derived rhodamine+ hematopoietic cells in the CHT were included here.
Fev regulation of HSC development is cell autonomous. (A) Transplantation scheme: rhodamine-labeled donor cells at the ventral marginal zone of cmyb:GFP transgenic donor embryos were transplanted into wild-type recipients at the blastula stage. (B) HSC reconstitution in the CHT of recipient embryos at 36 hpf. Green, cmyb:GFP+ cells; red, rhodamine. White arrows, GFP+ HSCs contributed by donor cells; light blue arrowheads, donor-derived GFP− hematopoietic cells. (C) Summary of transplantation results. Note that only host embryos with donor-derived rhodamine+ hematopoietic cells in the CHT were included here.
Fev is expressed and functions in purified human CB cells
Fev (also called Pet1 in mammals) is highly conserved in vertebrates.19,20 Therefore we asked whether fev is expressed and functions in human HSCs. Umbilical vein CB cells were used to address the issue. CB CD34+ cells have been well characterized as HSCs by in vitro colony-forming cell (CFC) assays.32-34 Here, we used this assay to assess functional roles of fev in human hematopoiesis.
We first examined the expression profile of fev in CB cells using RT-PCR. CB cells were flow-sorted as CD34+ and CD34− populations (Figure 6A). fev expression was consistently detected in CD34+ but not in CD34− cells for all samples examined in this study (Figure 6B).
fev is expressed and functions in human primitive HSCs. (A) Flow-sorting and purity detection of CD34+ and CD34− fractions from CD34-enriched CB cells. (B) RT-PCR detection of fev expression in fractions of (A). (C) Construction of fev-knockdown vectors (iFev or control virus [Ctr.V]). (D) Efficiency validation of fev-knockdown by Western-blot analysis in 293T cells permanently expressing fev. Mock: lysis of 293T cells. (E) The scheme of in vitro functional assays. CB CD34+ cells transduced with Ctr.V or iFev lentivirus were cultured in stem cell expansion medium for 5 d and then GFP+ cells were flow-sorted for CFC assay. (F) Flow-sorting and purity detection of GFP+ cells from 5-d cultured cells of (C). (G) RT-PCR detection of fev in the flow-sorted cells of (D), indicating the efficiency of fev knockdown. (H) Results of methylcellulose CFC assay of Ctr.V and iFev cells. iFev cells had an 8.5-fold decrease in total colonies (124.5 ± 29.9 vs 16.4 ± 3.4, n = 5 independent experiments, P = 0.0008). (I) The proportion of progenitors in total colonies, including erythroid CFU-E (colony forming unit-erythroid), myelo-monocytic CFU-G (colony forming unit-granulocyte), CFU-M (colony forming unit-macrophage), and CFU-GM (colony forming unit-granulocyte, macrophage), and mixed CFU-GEMM (colony forming unit-granulocyte, erythroid, macrophage, megakaryocyte) (n = 5 independent experiments; erythroid: P = 0.4; myelo-monocytic: P = 0.06; mixed: P = 0.0006). (J) Results of CFC replating of primary colonies of (F). iFev cells showed an 11.8-fold decrease in total colonies (32.7 ± 4.8 vs 2.8 ± 3.2, n = 5 independent experiments, P = 0.003). (K) Long time culture-initiating cell frequency of Ctr.V and iFev cells measured by limit-dilution assay. iFev cells showed a 12.7-fold decrease (60.5 ± 21.8 vs 4.8 ± 5.6, n = 5 independent experiments, P = 0.004). PC, positive control in HEK293T cells stably expressing fev; NC, non-template control.
fev is expressed and functions in human primitive HSCs. (A) Flow-sorting and purity detection of CD34+ and CD34− fractions from CD34-enriched CB cells. (B) RT-PCR detection of fev expression in fractions of (A). (C) Construction of fev-knockdown vectors (iFev or control virus [Ctr.V]). (D) Efficiency validation of fev-knockdown by Western-blot analysis in 293T cells permanently expressing fev. Mock: lysis of 293T cells. (E) The scheme of in vitro functional assays. CB CD34+ cells transduced with Ctr.V or iFev lentivirus were cultured in stem cell expansion medium for 5 d and then GFP+ cells were flow-sorted for CFC assay. (F) Flow-sorting and purity detection of GFP+ cells from 5-d cultured cells of (C). (G) RT-PCR detection of fev in the flow-sorted cells of (D), indicating the efficiency of fev knockdown. (H) Results of methylcellulose CFC assay of Ctr.V and iFev cells. iFev cells had an 8.5-fold decrease in total colonies (124.5 ± 29.9 vs 16.4 ± 3.4, n = 5 independent experiments, P = 0.0008). (I) The proportion of progenitors in total colonies, including erythroid CFU-E (colony forming unit-erythroid), myelo-monocytic CFU-G (colony forming unit-granulocyte), CFU-M (colony forming unit-macrophage), and CFU-GM (colony forming unit-granulocyte, macrophage), and mixed CFU-GEMM (colony forming unit-granulocyte, erythroid, macrophage, megakaryocyte) (n = 5 independent experiments; erythroid: P = 0.4; myelo-monocytic: P = 0.06; mixed: P = 0.0006). (J) Results of CFC replating of primary colonies of (F). iFev cells showed an 11.8-fold decrease in total colonies (32.7 ± 4.8 vs 2.8 ± 3.2, n = 5 independent experiments, P = 0.003). (K) Long time culture-initiating cell frequency of Ctr.V and iFev cells measured by limit-dilution assay. iFev cells showed a 12.7-fold decrease (60.5 ± 21.8 vs 4.8 ± 5.6, n = 5 independent experiments, P = 0.004). PC, positive control in HEK293T cells stably expressing fev; NC, non-template control.
To determine the function of fev in CB cells, a lentiviral, vector-mediated, knockdown system was constructed (Figure 6C). The short hairpin oligonucleotides of fev interference RNA (iFev) were cloned into the pLKO.1 lentiviral vector driven by a U6 promoter, and the expression was reported by enhanced GFP. Fev knockdown efficiency was validated by western blot in 293T-fev cells, which stably express fev protein (Figure 6D). We set up in vitro experiments as schemed in Figure 6E. CB CD34+ cells were transduced with iFev or control virus and cultured for 5 d under a well-characterized condition for maintaining self-renewal potential of stem cells33,35 before GFP+ cells were flow sorted with high purity (Figure 6F). RT-PCR analysis indicated that fev expression was efficiently knocked down in iFev-transduced cells but not in control virus-infected cells (Figure 6G). The purified cells were inoculated for in vitro functional assays. A CFC assay showed that fev knockdown led to an ∼8.5-fold reduction of total colony number (Figure 6H). Colonies were categorized into erythroid CFU-E, myelo-monocytic CFU-G, CFU-M, and CFU-GM, and mixed CFU-GEMM. Calculation of their proportions in total colonies showed that the primitive mixed CFCs were compromised more severely, whereas more mature cells were not significantly affected by fev knockdown (Figure 6I). CFC replating experiments suggested self-renewal defects in which reduction of colony-forming ability appeared to be more obvious when primary colony cells were collected and replated in new methylcellulose (Figure 6J). To further demonstrate this, we applied a quantitative limiting dilution analysis to evaluate the frequency of long time culture-initiating cells, the most primitive human progenitor assessable in vitro. We found that loss of fev reduced long time culture-initiating cell readout (Figure 6K). Taken together, these data imply that self-renewal of human primitive HSCs was impaired in the absence of Fev in vitro.
Discussion
In this study, we have shown that an ETS transcription factor, Fev (also called Pet1 in mammals), is required for HSC development and function. fev deficiency in zebrafish disrupted HSC emergence; therefore, the morphants had fewer T cells in the thymus. This phenotype was due to a cell-autonomous intrinsic defect in HSC specification. This effect is executed by a novel Fev-ERK signaling targeting the hemogenic endothelium-derived HSCs. Moreover, Fev is also expressed and functions in primitive HSCs in humans, indicating its conserved role in higher vertebrates.
During vertebrate embryogenesis, once the dorsal aorta is formed, a subset of ECs become specialized or “reprogrammed” with the expression of runx1, a pivotal transcription factor required for HSC generation.36 This specialized region in the ventral wall of dorsal aorta is called “hemogenic endothelium” from which the earliest HSCs are derived through an EHT process.1-3 Determining how the earliest HSCs arise from hemogenic endothelium and are maintained in adulthood is currently of great interest and may lead to new therapies. HSC fate decisions during development must be tightly controlled by both cell-intrinsic and -extrinsic regulatory mechanisms.4,11 So far, Runx1 is the only known cell-intrinsic regulator of hemogenic endothelium-derived HSCs and is required for only the initiation of EHT, not before or thereafter.36 Very recently, thrombin receptor, which is a prototypical G protein-coupled receptor, has been reported to negatively regulate the EHT process as a cell-extrinsic microenvironmental factor in mESCs and zebrafish.37 Thrombin receptor inhibition might increase the rate of HSC induction through accelerating EHT.37 Here, we demonstrated that loss-of-function of fev or erk2 attenuates, while gain-of-function up-regulates, the expression of runx1 specifically in the AGM, suggesting they are functionally upstream of runx1, and acts earlier than runx1 during hemogenic endothelium formation. The reduced ECs in the dorsal aorta and the following decrease in HSC numbers in fev morphants or mutants further indicate that Fev-ERK signaling can promote both the number of ECs that are specified to HSC fate and HSC induction through EHT.
Previous studies showed that fev is expressed in serotonergic neurons and required for serotonin synthesis in mammals.20 Serotonin is a monoamine neurotransmitter that has multiple functions in the central nervous system, gastrointestinal tract, and cardiovascular system.38 In humans, serotonin can enhance ex vivo expansion of CB CD34+ stem/progenitor cells, suggesting that serotonin might act as a paracrine factor to facilitate crosstalk between hematopoiesis and the neural system.38 However, our data, including fev expression in hematopoietic tissues in zebrafish and purified CD34+ CB cells in humans and blastula transplantation, strongly demonstrated that Fev acts cell autonomously to control HSC emergence through ERK signaling during the onset of definitive hematopoiesis. VEGF signaling has been reported to be an autocrine factor to regulate adult HSC survival in bone marrow.39 Our previous work has also shown that in Xenopus as well as in zebrafish, VEGF signaling is essential for HSC emergence both cell autonomously and non-cell autonomously.7,16 However, how the downstream core effector of VEGF signaling, ERK, regulates HSC development during vertebrate embryogenesis (for example, what are the direct target genes of ERK signaling in hemogenic endothelium and HSCs) remains to be determined.
In summary, the work presented here elucidates a novel role for fev, a previously uncharacterized ETS gene expressed in blood/vessels, which acts as a pivotal regulator of definitive HSC development in zebrafish and humans. In particular, our data reveal that Fev directly regulates ERK signaling during hemogenic endothelium formation and then HSC emergence, thus providing a new mechanism of how ETS factors control HSC development through a novel gene regulation. This provides some insights for new strategies to induce or expand transplantable HSCs in vitro or ex vivo for treatment of hematological diseases.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Drs Leonard Zon, David Traver, Sean Morrison, Elaine Dzierzak, and Tariq Enver for advice or critical reading of the manuscript. The authors thank Yi Zhang and Feng Gao for providing CB and T. Tanaka for providing hybridoma TM-β1. The authors are grateful to Fei Gao for help in transverse section and immunofluorescence analysis.
This work was supported by grants from the National Basic Research Program of China (2010CB945300, 2011CB943900, and 2012CB945101), the National Natural Science Foundation of China (30971678, 90919055), the Strategic Priority Research Program of the Chinese Academy of Sciences (XDA01010110), Science and Technology Commission of Shanghai Municipality (10PJ1406500), and the Medical Research Council.
Authorship
Contribution: L.W., T.L., L.X., Y.G., Y.W., and C.D. performed experiments; D.H. and F.L. designed the research, analyzed data, and wrote the manuscript; and G.-Q.C., S.L., R.P., and B.Z. participated in the preparation of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Feng Liu, State Key Laboratory of Biomembrane and Membrane Biotechnology, Institute of Zoology, Chinese Academy of Sciences, Beijing 100101, China; e-mail: liuf@ioz.ac.cn; and Dengli Hong, Department of Pathophysiology, Key Laboratory of Cell Differentiation and Apoptosis of Ministry of Education, Shanghai Jiaotong University School of Medicine, Shanghai 200025, China; e-mail: dlhong@shsmu.edu.cn.
References
Author notes
L.W., T.L., and L.X. contributed equally to this study.




![Figure 6. fev is expressed and functions in human primitive HSCs. (A) Flow-sorting and purity detection of CD34+ and CD34− fractions from CD34-enriched CB cells. (B) RT-PCR detection of fev expression in fractions of (A). (C) Construction of fev-knockdown vectors (iFev or control virus [Ctr.V]). (D) Efficiency validation of fev-knockdown by Western-blot analysis in 293T cells permanently expressing fev. Mock: lysis of 293T cells. (E) The scheme of in vitro functional assays. CB CD34+ cells transduced with Ctr.V or iFev lentivirus were cultured in stem cell expansion medium for 5 d and then GFP+ cells were flow-sorted for CFC assay. (F) Flow-sorting and purity detection of GFP+ cells from 5-d cultured cells of (C). (G) RT-PCR detection of fev in the flow-sorted cells of (D), indicating the efficiency of fev knockdown. (H) Results of methylcellulose CFC assay of Ctr.V and iFev cells. iFev cells had an 8.5-fold decrease in total colonies (124.5 ± 29.9 vs 16.4 ± 3.4, n = 5 independent experiments, P = 0.0008). (I) The proportion of progenitors in total colonies, including erythroid CFU-E (colony forming unit-erythroid), myelo-monocytic CFU-G (colony forming unit-granulocyte), CFU-M (colony forming unit-macrophage), and CFU-GM (colony forming unit-granulocyte, macrophage), and mixed CFU-GEMM (colony forming unit-granulocyte, erythroid, macrophage, megakaryocyte) (n = 5 independent experiments; erythroid: P = 0.4; myelo-monocytic: P = 0.06; mixed: P = 0.0006). (J) Results of CFC replating of primary colonies of (F). iFev cells showed an 11.8-fold decrease in total colonies (32.7 ± 4.8 vs 2.8 ± 3.2, n = 5 independent experiments, P = 0.003). (K) Long time culture-initiating cell frequency of Ctr.V and iFev cells measured by limit-dilution assay. iFev cells showed a 12.7-fold decrease (60.5 ± 21.8 vs 4.8 ± 5.6, n = 5 independent experiments, P = 0.004). PC, positive control in HEK293T cells stably expressing fev; NC, non-template control.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/122/3/10.1182_blood-2012-10-462655/4/m_367f6.jpeg?Expires=1767702714&Signature=Ii6CEFkXvVpIaaxfevZzLwapGTYfMoTX5Zlow59Bn3NyCGUDC-VxpS3vdFVODhJV75wpmtkO29KeDvsIk4Kn2s3g-yf2TP9BSG8L5GgtbgmfKmdeLd8vXaNAbiz1NSbGCIVI73FtqOC83Txn41KGuNPhNds3qs0YnRrhBLKzKNZXE2bpeklLW7cv6GhgMYlVh1ernomG2r1QzNxJ1RjyRVxv-Bz1ZJrlK-7lNt-aHQsHTtGjrrBEczy5JBqnZLMlIiK95ERRjykKT0v3AP9-ui2MHRfjoORLrdhjtWdIajDgtQ4kmtWnGTWGgaNIB~mRlRQhnJirgX-CoI2~H28Gmg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal